Current Pediatric Research
International Journal of Pediatrics
The onset of asymptomatic amiodarone-induced thyrotoxicosis in an adolescent male.
Pediatric Department, Pediatric Endocrinology, King Abdulaziz University Hospital, Saudi Arabia
- Corresponding Author:
- Abdulmoein Eid Al-Agha
Professor, Pediatric Endocrinology, King Abdulaziz University Hospital Pediatric Department
P.O.Box 80215 Jeddah 21589, Saudi Arabia
Tel: + 966 2 640 3841
Fax: + 966 2 6408353
E-mail: aagha@kau.edu.sa
Accepted date: July 27, 2018
The use of amiodarone is associated with many organ-specific side effects, of which thyroid gland dysfunction constitute up to 35% of them. Amiodarone-induced thyrotoxicosis can occur in either of two forms, type 1 or type 2, depending on whether there was a positive history of thyroid dysfunction prior to the use of amiodarone therapy or not, respectively. A unique underlying pathophysiology discerns one type from another, which is why early differentiation is pivotal to the rapid institution of appropriate therapy that is contingent on an accurate and correct diagnosis. We report a case of a 15-year-old adolescent with type 2 amiodarone-induced thyrotoxicosis, a rare presentation of thyrotoxicosis in children.
Keywords
Amiodarone, Thyrotoxicosis, Adolescent, Hyperthyroidism, Type 2.
Introduction
Thyroid disorders relating to the use of amiodarone therapy are considered the third most commonly encountered side effect constituting up to 35% of cases [1]. Amiodarone Induced Hypothyroidism (AIH) is more prevalent than Amiodarone Induced Thyrotoxicosis (AIT), which could be explained by the underlying mechanism through which amiodarone affects the thyroid gland. Amiodarone inhibits the uptake of both T4 and T3 into peripheral tissue as well as the monodeiodination of T4, which in turn, results in an overall decreased production of T3 [2-4]. Furthermore, amiodarone is accompanied by a high concentration of iodine, with 75 g to each 200 mg tablet. Subsequent deiodination through drug metabolism results in the daily release of approximately 10% as free iodide into the circulation [5]. However, in AIT, the mechanism by which this affects thyroid production has led to the classification of two different types, each with its own approach to investigation and management. In patients with pre-existing autoimmune thyroid dysfunction, a loss in normal thyroid auto-regulation and potentiation of autoimmunity has been observed, ushering a further increase in unregulated thyroid hormone synthesis [6]. This is especially pronounced in patients with a prior history of Grave's disease and has been termed the Jod-Basedow effect. These patients have been classified into type 1 AIT and have shown a better response to anti-thyroid medication [7]. On the other hand, patients with type 2 AIT suffer from a destructive thyroditis resulting in the increased release of preformed T3 and T4. This results from the direct cytotoxic effect of amiodarone on thyroid follicular cells, and will therefore respond best to the initiation of glucocorticoids as the main therapeutic approach [8]. A third type had been postulated, mixed AIT, in which features of both type 1 and type 2 are present and is therefore approached initially with combination therapy [1]. We report the presentation of a 15-year-old adolescent with type 2 AIT, as a rare presentation of thyrotoxicosis in children.
Case History
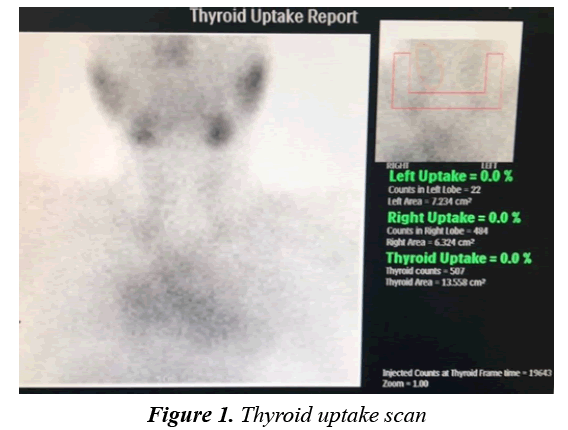
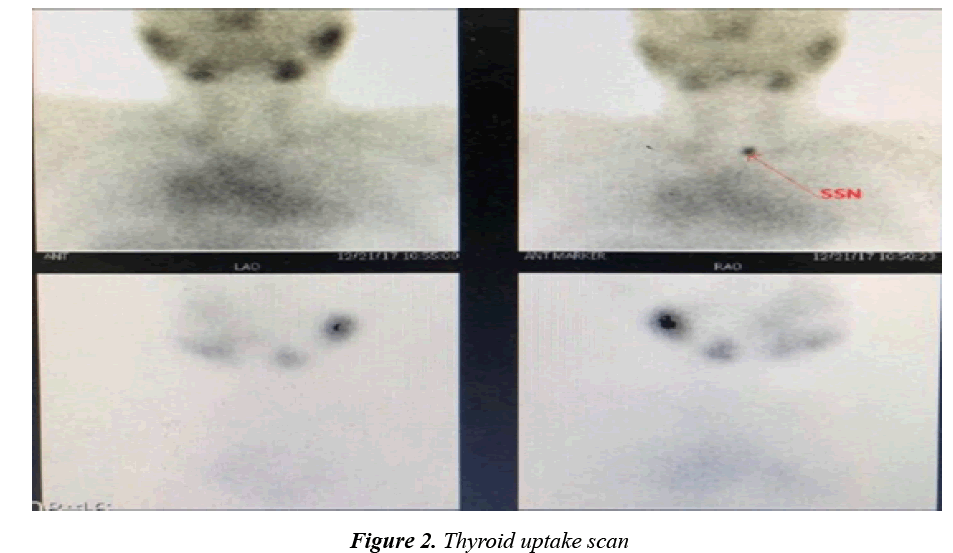
A fifteen-year-old adolescent male came for follow up due to his known case of complex congenital heart disease involving both Levo-Transposition of Great Arteries and Double Inlet Left Ventricle. He is post fontan procedure and balloon atrial septostomy done when he was two years of age. Since then, he has been suffering from recurrent attacks of supraventricular tachycardia and chest pain necessitating multiple admissions into the Emergency Department annually for a bolus of amiodarone at a rate of 5 mg/kg. At 11 years of age, he was started on amiodarone at a dose of 200 mg per day for a duration of 3 years until his presentation to the endocrinology clinic. He had suffered no signs or symptoms indicative of thyroid dysfunction such as weight loss, fever, irritability, tremors, diarrhoea, and resting tachycardia. He was haemodynamically stable without goiter, thyroidal tenderness, or lymphadenopathy, in addition, there was absence of exophthalmos, pretibial myxoedema and thyroid achropachy. He was found to be thyrotoxic as a result of routine screening of thyroid function (Table 1). It was confirmed that the patient had been euthyroid prior to the initiation of amiodarone therapy based on pretreatment assay of thyroid function, therefore raising the suspicion of type 2 AIT. The Radioiodine-131 scan had then confirmed the diagnosis by showing no uptake of contrast by the gland (Figures 1 and 2). A Colour Flow Doppler Sonography was ordered but came back normal and therefore was insignificant to the diagnosis. He was accordingly treated with prednisone 45 mg per day with complete normalisation of thyroid function by 2 months. He was maintained on treatment for a total of 3 months (Table 1) before finally tapering him off over the course of another 2 months at a rate of 5 mg reduction per week.
| fT3 (2.8-7.0) Pmol/L |
fT4 (12.0-22.0) Pmol/L |
TSH (0.27-4.2) UIU/L |
|
| At Admission | 9.7 | 45.7 | 0.005 |
| After 3 weeks of treatment | 2.7 | 18.7 | - |
| After 2 months of treatment | 4.7 | 22.7 | 1.19 |
| After 3 months of treatment | 3.4 | 18.8 | 1.30 |
Table 1: Thyroid Function Test
| Value | Reference Range | |
|---|---|---|
| Thyroid Peroxidase Antibody (IU/mL) | 0.44 | 0-5.61 |
| Thyroid Thyroglobulin Antibody (IU/mL) | 2.89 | 0-4.11 |
| Antinuclear Antibody | Titre 1:160 | Mild Positive |
| Antimitochondrial Antibody | Negative | Negative |
| Smooth Muscle Antibody | Moderately Positive | Moderately Positive |
| Parietal Cell Antibody | Negative | Negative |
| Double stranded Antibody (IU/mL) | 133.4 | 0-200 |
Table 2: Antibody Assay
Discussion
Although hyperthyroidism is a rare disorder in childhood, it still potentiates for serious consequences if not managed appropriately [9]. The most common cause for thyrotoxicosis in childhood is Graves’ disease, after which, consideration may be allowed for the possibility of subacute thyroiditis or the thyrotoxic phase variably preceding the onset of Hashimotos thyroiditis [10]. Other rare causes would include the administration of exogenous compounds such as thyroid hormones or iodide constituents.
The clinical features of AIT are similar to those of thyrotoxicosis of any aetiology and include weight loss, heat intolerance, fatigue, muscle weakness, diarrhoea, nervousness, anxiety and palpitations. Therefore, it would necessitate the exclusion of the more common and distinguishable differentials such as autoimmune induced thyrotoxicosis. This patient did not have any family history significant for autoimmunity or thyroid disease, nor had he presented with any of the clinaical hallmarks that would render Graves disease likely, such as exophthalmos, pretibial myxoedema, and or thyroid achropachy. Furthermore, the positive history of chronic amiodarone administration has rendered the priority to AIT as the more likely differential. Nevertheless, a thyroid autoantibody panel had been ordered (Table 2), and had similarly come back negative.
Both types of AIT present similarly, and therefore cannot be differentiated clinically. Moreover, the management of each type is entirely different based on the underlying pathogenesis and therefore the diagnosis of the correct type of AIT is imperative. Based on the available literature, Colour Flow Doppler Sonography (CFDS) has yielded a definitive diagnosis in 80% of the cases [3,11]. In patients with type 2 AIT, CFDS results are expected to show compatibility with absent vascularity and evidence of gland destruction [8]. However, the CFDS in this patient had come back normal.
Another useful test include a radioiodine-131 scan, which is usually normal or show an increased uptake in type 1 due to the glands hyperactivity, whereas there is no uptake in type 2 as a result of the damage and destruction of the thyroid tissue [12]. The scan had come compatible with a type 2 AIT, showing the entailing 0% contrast uptake by the thyroid gland. Together with his history of normal thyroid function test results prior to amiodarone intervention and dramatic improvement upon the initiation of steroids, the diagnosis of type 2 AIT is consolidated.
It is worthy to mention that he had presented in a vitally stable state without any clinical indication of an underlying thyroid disorder despite his dangerously elevated levels of T3. However, an explanation could be provided by the fact that the general symptoms associated with thyrotoxicosis in this patient could have been masked due to the underlying Beta-blocking effects of amiodarone [1]. One study had reported that out of the 15.9% who suffered from the thyrotoxic variant of amiodarone related thyroid dysfunction, only 5.3% presented with clinical thyrotoxicosis while the remaining 10.6% had no suggestive clinical picture [13]. That same study also revealed that only 49.2% of all cardiologists demonstrated acceptable follow up of thyroid function in patients who were undertaking amiodarone therapy, which is to screen them on a trimonthly basis during treatment and at least up to one year after discontinuation [8]. This is a cause for concern as the risk accompanied by thyrotoxicosis is more critical in patients with an underlying significant cardiovascular disease, and more patients than not suffer a premonitory clinical picture suggestive of thyroid dysfunction. By this we gather that the more frequent diagnosis of AIT would rely tremendously on routine testing of thyroid function rather than symptomatology. Another case had detailed the onset of AIT one year after a discontinuation of amiodarone therapy [14], elucidating the importance of continuing follow up further after treatment is stopped. This is due to the prolonged elimination half-life of amiodarone, which can extend between 50 to 100 days. The effects of amiodarone therapy on the thyroid gland may be witnessed from just a few weeks after the initiation of therapy up to 3 years after discontinuation [15]. In this case, it had presented after three years of continued use.
In conclusion, AIT is recognised as a diagnostic and therapeutic challenge. Considering the different underlying pathological process to each type and therefore different associated therapeutic approach, a clinical conundrum surfaces when trying to confidently diagnose patients with an unknown prior thyroid function status. Therefore we recommend all paediatric cardiologists to perform a thyroid function test before the initiation of amiodarone therapy in order to avoid this challenge. Furthermore, we strongly reinforce the recommendation of trimonthly screening for thyroid dysfunction for as long as the patient is on amiodarone therapy and for at least one year after discontinuation.
The limitation of this study is the inability to generalise and determine a direct and accurate cause-and-effect relationship, as this is a single patient based case report.
Consent for Publication
Consent was obtained from the patients’ legal guardian for this publication.
References
- Narayana SK, Woods DR, Boos CJ. Management of amiodarone-related thyroid problems. Therapeutic Advances in Endocrinology and Metabolism 2011; 2: 115-126.
- Harjai KJ, Licata AA. Effects of amiodarone on thyroid function. Annals of Internal Medicine 1997. 126; 63-73.
- Bogazzi F, Martino E, Dell’Unto E, et al. Thyroid color flow doppler sonography and radioiodine uptake in 55 consecutive patients with amiodarone-induced thyrotoxicosis. J Endocrinol Invest. 2003; 26: 635-640.
- Chiovato L, Martino E, Tonacchera M, et al. Studies on the in vitro cytotoxic effect of amiodarone. Endocrinology 1994; 134: 2277-2282.
- Rushton P. Amiodarone and the thyroid. C J Geriatr Med 2004; 6: 20-22.
- Braverman LE, Ingbar SH, Vagenakis AG, et al. Enhanced susceptibility to iodide myxedema in patients with hashimoto’s disease. J Clin Endocrinol Metab 1971; 32: 515-521.
- Cardenas GA, Cabral JM, Leslie CA. Amiodarone induced thyrotoxicosis: Diagnostic and therapeutic strategies. Cleve Clin J Med. 2003; 70: 624-626.
- Frcpc WT, Frcpc RLH, Tsang W, et al. Amiodarone-induced amiodarone-induced thyrotoxicosis : A review 2009; 25: 421-424.
- Lavard L, Ranlov I, Perrild H, et al. Incidence of juvenile thyrotoxicosis in Denmark, 1982-1988. A nationwide study. Eur J Endocrinol 1994; 130: 565-568.
- Léger J, Carel JC. When and how to treat 2013; 5: 50-56.
- Eaton SEM, Euinton HA, Newman CM, et al. Clinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: Role of colour-flow Doppler sonography. Clin Endocrinol 2002; 56: 33-38.
- Martino E, Aghini-Lombardi F, Lippi F. Twenty-four hour radioactive iodine uptake in 35 patients with amiodarone associated thyrotoxicosis. J Nucl Med 1985; 26: 1402-1407.
- Tavares ABW, Paula SK de, Vaisman M, et al. Amiodarone and thyrotoxicosis: Case reports. Arq Bras Cardiol 2010; 95: e122-124.
- Khan A, Puttanna A, Raskauskiene D. Amiodarone-induced thyrotoxicosis: Type 1 or type 2? BMJ Case Rep 2014; 2014: 2-4.
- Pollak P. Population pharmacokinetics of long-term oral amiodarone therapy. Clin Pharmacol Ther. 2000; 67: 642-652.