Current Pediatric Research
International Journal of Pediatrics
The frequency of inherited metabolic and endocrine disorders in the eastern and north-western Jawf provinces of Saudi Arabia: Four years data from the newborn screening department, Ministry of Health, Dammam.
1The Newborn Screening Department, Regional Laboratory and Blood Bank, Dammam Medical Complex, Dammam, Kingdom of Saudi Arabia.
2ExpressMed Laboratories, Janabiyah, Kingdom of Bahrain, Bahrain.
- Corresponding Author:
- Randa Alratrout
Consultant Chemical Pathologist
Head of Newborn Screening Department
Regional Laboratory and Blood Bank
Dammam Medical Complex, Dammam, Kingdom of Saudi Arabia
Tel: +966 505863372
E-mail: ranratrout@yahoo.com
Accepted date: December 31, 2017
We investigated the incidence of seventeen inherited metabolic and endocrine disorders in the Eastern and Jawf Provinces of Saudi Arabia as part of the Saudi National Newborn Screening Program. A retrospective study was conducted between 1st January 2013 and 31st July 2017; all newborns from 20 MOH hospitals in Eastern and (North-western) Jawf Provinces were screened during the study period. Heel prick dry blood spot samples were obtained from all newborns for biochemical and immunoassay testing. Results were compared with other regional, national, and international studies. The total number of newborn babies screened was 199143, of which 264 were positive. This represents an incidence of 1:754.3 live births. The most frequently detected were very long chain acyl-CoA dehydrogenase deficiency (62 cases out of 199143, incidence 1:3211) followed by congenital hypothyroidism (59 out of 199143, incidence 1:3375) and congenital adrenal hyperplasia (25 out of 199143, incidence 1:7965), phenylketonuria (18 out of 199143, incidence 1:11063), and the least common was beta-ketothiolase deficiency (0 out of 199143, incidence 0). Conclusion: The incidence of these seventeen inherited metabolic and endocrine disorders in Eastern and Jawf Provinces is amongst the very highest in the world, attributable to high rates of consanguineous marriage. The incidence of VLCAD is especially high and largely restricted to the Alsherarat tribe in Jawf Province. Of all the towns in Eastern and Jawf Provinces Al-Ahsa has the highest incidence of these inherited disorders. Comparison with other national studies uncovered geographical differences in the incidence of certain inherited metabolic disorders.
Keywords
Inherited metabolic, Inherited endocrine, Newborn screening Program, incidence, Eastern, Jawf, Province, Consanguineous, Consanguinity.
Abbreviations
ASL: Argininosuccinate Lyase Deficiency; BKT: Beta-Ketothiolase Deficiency; BTD: Biotinidase Deficiency; CAH: Congenital Adrenal Hyperplasia; CH: Congenital Hypothyroidism; CIT: Citrullinemia; DBS: Dried Blood Spot; GA-1: Glutaric Academia Type 1; GALT: Galactosemia; GSP: Genetic Screening Processor; HMG: 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency; IEM: Inborn Error of Metabolism; IVA: Isovaleric Academia; MCAD: Medium-Chain Acyl-CoA Dehydrogenase Deficiency; 3-MCC: 3-Methylcrotonyl-CoA Carboxylase Deficiency; MMA: Methylmalonic Academia; MOH: Ministry of Health; MSUD: Maple Syrup Urine Disease; NNBS: National Newborn Screening Program; NBS: Newborn Screening; PA: Propionic Academia; PKU: Phenylketonuria; TMS: Tandem Mass Spectrometry; VLCAD: Very Long Chain Acyl-CoA Dehydrogenase Deficiency
Introduction
The aim of Newborn Screening (NBS) is the early detection and treatment of clinically important disorders such as Inborn Errors of Metabolism (IEM) in order to minimize morbidity and mortality in early childhood. In recent times, with the development of electrospray Tandem Mass Spectrometry (TMS), it has become possible to use a single test to screen for a wide range of rare but clinically significant disorders that have not been screened for previously [1]. Requiring only a few drops of blood from the newborn, TMS allows for rapid simultaneous analysis and detection of a broad variety of metabolic diseases including amino acid, organic acid and fatty acid defects, in addition to endocrine disorders and even hemoglobinopathies [1-3].
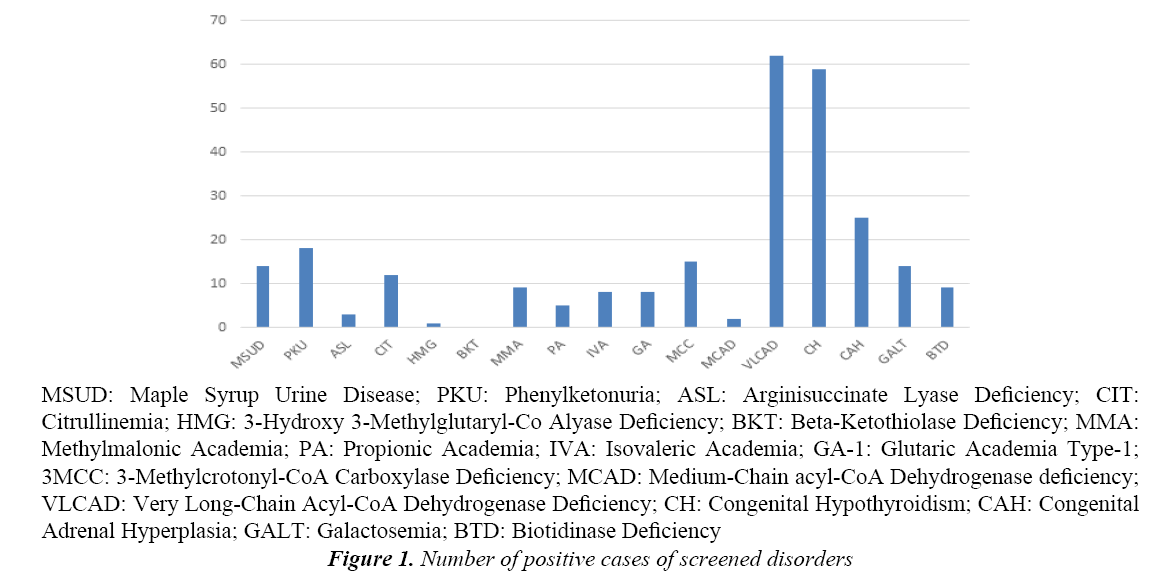
The Saudi National Newborn Screening Program (NNBS)4 was launched in August 2005 by the Saudi Ministry of Health (MOH) with its main objective to assess the incidence of the seventeen most common IEM and inherited endocrine disorders nationwide (Tables 1 and 2 and Figure 1).
| Disorder | Marker | Number of Positive Cases | % Positive Cases |
|---|---|---|---|
| Amino Acid Disorders | |||
| PKU MSUD Citrullinemia ASL |
Phe,Phe/Tyr ratio Leu/Ileu,Valine Citrulline Citrulline, ASA |
18 14 12 3 |
6.8 5.3 4.5 1.1 |
| Organic Acids Disorders | |||
| 3-MCC MMA PA IVA GA-I HMG BKT |
C5OH C3 C3 C5 C5DC, C5DC/C5OH, C5DC/C8 C5OH,C6DC C5:1,C5OH |
15 9 5 8 81 0 |
5.6 3.4 1.8 3.0 3.00.3 0 |
| Fatty Acid Oxidation Disorders | |||
| MCAD VLCAD |
C6, C8, C10 C14, C14:1, C14:2, C14:1/C16 ratio |
2 62 |
0.75 23.4 |
| Congenital Hypothyroidism | |||
| TSH, T4 | 59 | 22.3 | |
| Congenital Adrenal Hyperplasia | |||
| 17 hydroxy progesterone | 25 | 9.4 | |
| GALT | |||
| GALT enzyme | 14 | 5.3 | |
| Biotidinase Deficiency | |||
| Biotidinase enzyme | 9 | 3.4 | |
Table 1: Frequencies of inherited metabolic and endocrine disorders in the study group (2013-2017) and markers tested for each disorder
| No. | Disease | No. of positive cases | Incidence |
|---|---|---|---|
| 1 | VLCAD | 62 | 1:3211 |
| 2 | CH | 59 | 1:3375 |
| 3 | CAH | 25 | 1:7965 |
| 4 | PKU | 18 | 1:11063 |
| 5 | 3-MCC | 15 | 1:13276 |
| 6 | GALT | 14 | 1:14224 |
| 7 | MSUD | 14 | 1:14224 |
| 8 | CIT | 12 | 1:16595 |
| 9 | MMA | 9 | 1:22127 |
| 10 | BTD | 9 | 1:22127 |
| 11 | IVA | 8 | 1:24892 |
| 12 | GA-1 | 8 | 1:24892 |
| 13 | PA | 5 | 1:39828 |
| 14 | ASL | 3 | 1:66381 |
| 15 | MCAD | 2 | 1:99571 |
| 16 | HMG | 1 | 1:199143 |
| 17 | BKT | 0 | 0 |
| Total | 264 | 1:754 |
Table 2: Incidence of each screened disorder
The NNBS Program is organized in three phases. As part of phase 2 of the NNBS, data on newborn screening for these seventeen disorders are being obtained for the Eastern Province of Saudi Arabia. Newborn screening data obtained in the eastern and (north-western) Jawf provinces between January 2013 and July 2017 are presented and discussed in this paper.
Methods
Data on newborn screening collected between 1 January, 2013 and 31 July, 2017 were retrospectively analyzed.
The 17 disorders screened (as decided by the NNBS committee, Tables 1 and 2 and Figure 1) were: the Aminoacidopathies Phenylketonuria (PKU), Argininosuccinate Lyase Deficiency (ASL), Maple Syrup Urine Disease (MSUD) and Citrullinemia (CIT); The Organic Acid Disorders Propionic Acidemia (PA), Methylmalonic Acidemia (MMA), Glutaric Academia Type 1 (GA-1), Isovaleric Academia (IVA) and 3-Methylcrotonyl-CoA Carboxylase Deficiency (3- MCC); The fatty acid oxidation defect disorders Medium- Chain acyl-CoA Dehydrogenase Deficiency (MCAD) and Very Long Chain acyl-CoA Dehydrogenase Deficiency (VLCAD); The ketogenesis and ketolysis defects 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency (HMG) and Beta-Ketothiolase Deficiency (BKT); The Carbohydrate Disorder Galactosemia (GALT); The endocrine disorders Congenital Hypothyroidism (CH) and Congenital Adrenal Hyperplasia (CAH); and a vitamin responsive disorder Biotinidase Deficiency (BTD). These disorders can be divided into inherited endocrine disorders (CH and CAH) and inherited metabolic disorders (all the others).
All newborns from 20 MOH hospitals in Eastern and (North-western) Jawf (also spelt Jouf) Provinces were screened during the study period (and this practice continues to the present day). For each neonate a blood sample was collected by heel prick as a dried blood spot (DBS) on filter paper 24 h-5 days after birth, and analyzed by tandem mass spectrometry (API 3200 LC/MS/MS system by AB Sciex) and flurometric immunoassay (2021 Genetic screening processor – GSP –by PerkinElmer).
A PerkinElmer 226 collection device was used to collect and transport blood specimens (DBS) to the laboratory. NHS guidelines were followed for heel prick sampling, and blood spots were collected on PerkinElmer 226 filter paper cards in accordance with the manufacturer’s instructions. The sample collection card for each test subject was filled out with pertinent demographic details for neonate and mother, i.e., name, d.o.b. ID numbers.
Cut-off levels for each analyte tested were established using population data and data obtained from retrospective samples with proven disorders (Table 3).
| Method | Analyte | Cut-off for repeat sample ≥ |
|---|---|---|
| kkkMSMS Aminoacids |
Phe Phe:Tyr Val Leu,IsoLeu Leu:Ala Leu:Phe Cit Cit:Arg ASA |
150 2 300 290 1.28 5 85 15 0.6 |
| Acylcarnitines | C5OH C5:1 C3 C3:C2 C3:C16 C5 C5:C2 C5:C3 C5DC C6 C8 C10 C8:C10 C14 C14:1 C14:1C16 |
0.8 0.5 7 0.2 2 1.5 0.05 0.7 0.8 0.5 0.35 0.37 3 1:1 0.75 0.25 |
| GSP (Genetic Screening Processor) |
17-OH TSH GALT BTD |
55 20 3.4 39.4 |
Table 3: Cut-off values per volume of whole blood
Organic acids and amino acids were measured by MS/ MS and reconfirmed by MS/MS or amino acid analyzer or gas chromatography (urine samples). GSP was used for measuring biotidinase enzyme and GALT enzyme and reconfirmed by GSP.
Any abnormal result required confirmation by testing of a repeat DBS sample for that neonate and further confirmation by urine analysis. A second abnormal result (on the retest) was then regarded as sufficient evidence of a “positive” case. For positive cases the result was immediately communicated by Whatsapp, telephone and fax to the referring hospital so that any management measures could be started urgently.
Results
During the period 1 January, 2013-31 July, 2017 the total number of newborn babies screened was 199143, of which 264 were positive. This represents an incidence of 1:754.3 and cumulative incidence of 132.5 per 100,000 live births.
Tables 1 and 2 and Figure 1 show the frequency and incidence of each of the 17 screened disorders studied.
Out of the 17 disorders screened the most frequently detected were very long chain acyl-CoA dehydrogenase deficiency, VLCAD (62 cases out of 199143, incidence 1:3211) followed by congenital hypothyroidism, CH (59 out of 199143, incidence 1:3375) and congenital adrenal hyperplasia, CAH (25 out of 199143, incidence 1:7965). With the exception of the inherited metabolic disorder VLCAD, the inherited endocrine disorders as a group (CH and CAH) were the most frequently detected. Of the remaining disorders phenylketonuria (PKU) was the most frequent (18 out of 199143, incidence 1:11063), and the least common was beta-ketothiolase deficiency, BKT (0 out of 199143, incidence 0), all the other disorders being intermediate in frequency between these.
Table 4 shows a comparison of the current study findings with other similar regional and international studies. A study from Riyadh, Saudi Arabia, in 2017 [4] investigated 16 disorders, all of which were also covered in our current study, our seventeenth disorder being VLCAD. In their study with sample size 775,000 they found an overall incidence of their screened 16 inherited disorders of 1:1443, cumulative incidence 95.8/100,000, the most frequently detected disorders being CH and CAH with incidences of 1:7175 and 1:7908, respectively. PA was their most common inherited metabolic disorder with an incidence of 1:14090. Another study from Riyadh in 2016 looked at samples from 110601 neonates in which 14 inherited disorders investigated were common to our current study [5]. They did not include the inherited endocrine disorders CH and CAH in their study, but theirs was the only report–other than our own–that investigated the incidence of VLCAD. They found an overall incidence of their screened disorders of 1:1813, cumulative incidence 55.1/100,000, the most frequently detected disorders being PKU and PA with an incidence of 1:12289 each. They found VLCAD to have an incidence of 1:36867 (while the VLCAD incidence in our study was 1:3211). Data from two other studies from the Eastern Province of Saudi Arabia, both examining smaller sample sizes than our own, are also shown in Table 4 [6,7]. The Saudi Aramco study reported in 2010 looked at samples from 165530 neonates in which 12 inherited disorders investigated were common to our current study [6]. They did not include the inherited endocrine disorders CH and CAH. The overall incidence of their screened disorders was 1:2095, cumulative incidence 47.7/100,000, the most frequently detected disorders being PKU and GALT with an incidence of 1:10345 each. The study from Al Ahsa reported in 2011 looked at samples from 37168 neonates in which 11 inherited disorders investigated were common to our current study including CH and CAH [7]. The overall incidence of their screened disorders was 1:701.2, cumulative incidence 142.6/100,000, the most frequently detected disorder being 3-MCC with an incidence of 1:2859 followed by CH and biotinidase deficiency (BTD) each with an incidence of 1:3097. Data of two studies from outside Saudi Arabia (British Columbia, Canada and Oman) are also shown in Table 4 [8,9]. The Joshi et al. [9] study reported in 2002 looked at samples from 127500 neonates in which 9 inherited disorders investigated were common to our current study. They did not include the inherited endocrine disorders CH and CAH. The overall incidence of their screened disorders was 1:6071, cumulative incidence 16.7/100,000, the most frequently detected disorder being PPA with an incidence of 1:31875 followed by PKU, ASL, CIT and HMG each with an incidence of 1:42500. The Canadian study reported in 2000 looked at samples from 1142912 neonates in which 12 inherited disorders investigated were common to our current study [10]. They did not include the inherited endocrine disorders CH and CAH. The overall incidence of their screened disorders was 1:5218, cumulative incidence 19.1/100,000, the most frequently detected disorder being PKU with an incidence of 1:13289 followed by GALT with an incidence of 1:71432.
| Program | Current study Saudi Arabia (Eastern & Jawf) |
Saudi Arabia (Central & Western [6]) | Saudi Arabia (Central [7]) | Saudi Aramco Saudi Arabia (Eastern [8]) |
British Colombia Canada [10] |
Al Ahsa Saudi Arabia (Eastern [9]) | Oman [11] |
|---|---|---|---|---|---|---|---|
| Study Years | 2013-2017 | 2005-2012 | 2003-2016 | 1983-2008 | 1969-1996 | 2006-2009 | 1998-2006 |
| Population | 199143 | 775000 | 110601 | 165530 | 1142912 | 37168 | 127500 |
| No. of Positive Cases | 264 | 743 | 61 | 79 | 219 | 53 | 21 |
| General Incidence | 13:34.3 | 0.76597222 | 1.30069444 | 1.49652778 | 3.66527778 | 12:41.2 | 4.25763889 |
| Cumulative Incidence Rate/100000 LB | 132.5 | 95.8 | 55.1 | 47.7 | 19.1 | 142.6 | 16.7 |
| Aminoacidopathy | |||||||
| PKU | 1:11063 (18) | 1:14245 | 1:12289 (9) | 1:10345 (16) | 1:13289 (86) | 1:37168 (1) | 1:42500 (3) |
| MSUD | 1:14224 (14) | 1:15816 | 1:22120 (5) | 1:13794 (12) | 1:571456 (2) | Not screened | 1:127500 (1) |
| Urea Cycle Disorders | |||||||
| ASL | 1:66381 (3) | 1:16847 | 1:13825 (8) | 1:27588 (6) | 1:380970 (3) | Not screened | 1:42500 (3) |
| CIT | 1:16595 (12) | 1:17222 | 1:27650 (4) | 1:27588 (6) | 1:380970 (3) | 1:37168 (1) | 1:42500 (3) |
| Organic Acids Disorders | |||||||
| HMG | 1:199143 (1) | 1:55357 | Not screened | Not screened | Not screened | Not screened | 1:42500 (3) |
| BKT | 0 | 1:193750 | 1:110601 (1) | Not screened | 1:571456 (2) | Not screened | Not screened |
| 3MCC | 1:13276 (15) | 1:16847 | 1:36867 (3) | 1:55176 (3) | Not screened | 1:2859 (13) | Not screened |
| GA-Type I | 1:24892 (8) | 1:32291 | 1:36867 (3) | 1:55176 (3) | 1:380970 (3) | 1:37168 (1) | Not screened |
| PA | 1:39828 (5) | 1:14090 | 1:12289 (9) | 1:27588 (6) | 1:380970 (3) | 1:37168 (1) | 1:31875 (4) |
| MMA | 1:22127 (9) | 1:15500 | 1:15800 (7) | 0 | 1:571456 (2) | 1:18586 (2) | 1:127500 (1) |
| IVA | 1:24892 (8) | 1:29807 | 1:110601 (1) | 1:27588 (6) | 1:571456 (2) | Not screened | 1:127500 (1) |
| FA Disorders | |||||||
| MCAD | 1:99571 (2) | 1:23484 | 1:110601 (1) | 1:82765 (2) | 1:571456 (2) | 1:7433 (5) | Not screened |
| VLCAD | 1:3211 (62) | Not screened | 1:36867 (3) | Not screened | Not screened | Not screened | Not screened |
| Congenital Hypothyroidism | 1:3375 (59) | 5.02430556 | Not screened | Not screened | Not screened | 1:3097 (12) | Not screened |
| Congenital Adrenal Hyperplasia | 1:7965 (25) | 5.53333333 | Not screened | Not screened | Not screened | 1:12389 (3) | Not screened |
| GALT | 1:14224 (14) | 1:14245 | 1:27650 (4) | 1:10345 (16) | 1:71432 (16) | 1:18584 (2) | 1:63750 (2) |
| Biotidinase deficiency | 1:22127 (9) | 1:21527 | 1:36867 (3) | 1;55176 (3) | 1:228582 (5) | 1:3097 (12) | Not screened |
Table 4: Comparison of the frequency of inherited metabolic and endocrine disorders with other retrospective population based studies
Table 5 shows the geographic distribution of the positive cases. Cases of the three commonest disorders in our study- VLCAD, CH, CAH – were from both Eastern and Jawf Provinces, while all cases of PKU were from Eastern province. Fifty-three of the 59 cases of CH were from Eastern Province (Al Ahsa 3, Dammam 11, Hafer 6, Qatif 3, Jobail 3) and 6 CH cases were from Jawf Province (Sakakah 4, Dumat Al-Jandal 2). Twenty-four of the 25 cases of CAH were from Eastern Province (Al Ahsa 9, Dammam 7, Hafer 7, Qatif 1) and 1 CAH case was from Jawf Province (Sakakah). All 18 cases of PKU were from Eastern Province (Dammam 10, Qatif 5, Al Ahsa 2, Hafer 1). Fifty-one of the 62 cases of VLCAD were from Northern Jawf Province (Tabargal 32, Dumat Al-Jandal 15, Sakakah 4) and 11 VLCAD cases were from Eastern Province (Dammam 5, Khafji 2, Qaif 1, Al-Ahsa 1, Hafer 1, Jubail 1).
| # | Diseases | Eastern Provence (Region) |
AlJawf Provence (Region) |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dammam | Qatif | Al Ahsa | Qarea alolya | Kufji | Hafr albaten |
Jubail | Sakakah | Dumat Al-Jandal | Tabargal | |||
| 1 | MSUD | 3 | 1 | 10 | 14 | |||||||
| 2 | PKU | 10 | 5 | 2 | 1 | 18 | ||||||
| 3 | ASL | 1 | 1 | 1 | 3 | |||||||
| 4 | CIT | 4 | 1 | 5 | 2 | 12 | ||||||
| 5 | HMG | 1 | 1 | |||||||||
| 6 | BKT | - | ||||||||||
| 7 | MMA | 8 | 1 | 9 | ||||||||
| 8 | PA | 4 | 1 | 5 | ||||||||
| 9 | IVA | 6 | 1 | 1 | 8 | |||||||
| 10 | GA | 3 | 5 | 8 | ||||||||
| 11 | MCC | 2 | 13 | 15 | ||||||||
| 12 | MCAD | 2 | 2 | |||||||||
| 13 | VLCAD | 5 | 1 | 1 | 2 | 1 | 1 | 4 | 15 | 32 | 62 | |
| 14 | CH | 11 | 3 | 3 0 | 6 | 3 | 4 | 2 | 59 | |||
| 15 | CAH | 7 | 1 | 9 | 7 | 1 | 25 | |||||
| 16 | GALT | 4 | 1 | 3 | 1 | 1 | 3 | 1 | 14 | |||
| 17 | BTD | 1 | 6 | 1 | 1 | 9 | ||||||
| Total | 72 | 14 | 88 | 1 | 2 | 18 | 4 | 13 | 19 | 33 | 264 | |
Table 5: Geographic distribution of positive cases
In terms of the geographical distribution of these inherited disorders as a group, the town/city with the highest number of cases was Al-Ahsa (88/264) followed by Dammam (72/264) and Tabargal (33/264).
Discussion
This study provides the largest set of results for the incidence of inherited metabolic and endocrine disorders in the Eastern and North-western Jawf Provinces of Saudi Arabia (199143 new-borns screened). Retrospective analysis of the results from all newborns from 20 MOH hospitals in Eastern and Jawf Provinces during the period 1 January 2013 to 31 July 2017 was carried out. The screening of this cohort formed part of phase 2 of The Saudi National Newborn Screening Program (NNBS), a program launched in August 2005 and expected in three phases to cover the entire Kingdom of Saudi Arabia. The largest region is Eastern Province covering an area of approximately 673 000 sq. km (Figure 2). The population of Saudi Arabia is about 31 million with a growth rate of 3% and live births are estimated at 569 000 neonates per year [10,11].
The current study examined data from 199143 neonates. The general incidence of inherited metabolic and endocrine disorders was 1:754.3 and the most frequently detected disorders were VLCAD, CH, CAH and PKU.
Other similar studies from the eastern province of Saudi Arabia include the Saudi Aramco study (165530 newborns screened) and the Al-Ahsa study (37168 newborns screened) [6,7]. The current study investigated a higher number of newborns (1,99,143) than either of these two studies, our cohort numbers being significantly higher than the Al-Ahsa study and higher but comparable to the Saudi Aramco study.
Comparison of data from the seven studies shown in Table 4 demonstrates that the incidence of inherited metabolic and endocrine disorders is significantly higher in Saudi Arabia than other parts of the world (regional and wider international). Furthermore, within Saudi Arabia, there appears to be a geographical difference in the incidence of these disorders with higher incidences seen in the Eastern and Northern-western Jawf Provinces (current study and Al Ahsa study in particular) compared to Central and Western Provinces (two studies from Riyadh) [4,5]: The approximate incidence of 1:700 in Eastern and Northern Jawf Provinces as revealed by the current study and the Al Ahsa study is significantly higher than the incidence of 1:1043 for Central and Western Provinces [4,7]. In comparison with data from countries outside the Gulf and Middle East, our data shows that the incidence of these disorders in Eastern and Jawf Provinces of Saudi Arabia (1:754) is considerably higher than Canada (1:5218), Australia (1:4500) and USA (1:1792) [8,12,13]. In comparison with other Gulf countries our figures are again significantly higher than Oman (1:6071), Qatar (1:1327) and UAE (1:1047) [9,14,15].
From these data it can be concluded that the incidence of inherited metabolic and endocrine disorders in Saudi Arabia and in particular the Eastern and North-western Jawf Provinces of Saudi Arabia is among the highest in the world (as compared with published data from other countries).
The reason for this can be attributed in large part to consanguineous marriages between relatives as close as first cousins. Consanguineous marriages are common in Saudi society and are estimated at 51 to 56% of which around 30% are between first cousins [16-18]. As a result of these high rates of consanguinity there is a high prevalence of autosomal recessive genetic disorders in Saudi Arabia including IEMs.
Explaining the high rates of consanguinity in Saudi Arabia is the fact that Saudi Society is and has historically always been divided on the lines of tribes and clans. As a consequence of this there is a strong tendency for marriages to occur between same tribe/same clan members hence concentrating abnormal alleles in multiple small cohorts of the population and increasing the incidence of inherited/genetic disorders.
As borne out by our data there is a clear and significant difference between the incidence of these inherited disorders within Saudi Arabia, with a greater incidence in Eastern and North-western Jawf Provinces compared to other provinces notably Central and Western Provinces. We propose that this can be explained by two main reasons: a. increased consanguinity as a result of even greater and complex tribal divisions leading to higher levels of concentration/pooling of abnormal alleles in even more small cohorts of the population than in the rest of Saudi Arabia; b. greater religious diversity in Eastern Province compared to the rest of Saudi Arabia. Saudi Arabia is mostly a society of Sunni Muslims, as many as 97% of Saudi citizens are Sunni [19]. The remaining 3% of Saudi citizens belong to the minority Shia (Shiite) sect. However, in Eastern province the proportion of Shia is much higher around 24%, and in some areas such as Al Ahsa and Qatif Shia Muslims are in the majority [19]. This in turn provides an additional tier to consanguinity in the Eastern Province, as marriages will not only be within tribes but also within religious sects leading to higher levels of concentration/pooling of abnormal alleles in even more small cohorts of the population than in the rest of Saudi Arabia which has a larger Sunni majority (and less diversity of religion). Sunni-Shia mixed marriages are rare. Based on this within Eastern Province one would expect to see higher incidences of inherited genetic disorders in towns/areas with significant Shia populations and this is indeed the case, especially in Al Ahsa the single town with the largest number of positive cases (88/264, 33%).
The Oman data are very interesting. Oman, even though it is a geographical neighbor of Saudi Arabia and like Saudi Arabia is an Arab country, has a much lower incidence of IEMs. In part, this can be attributed to lower levels of consanguinity in Oman compared to Saudi Arabia. While the proportion of consanguineous marriages among Saudis is as high as 56% the figure for Omanis is around 30% and even then most are between relatives more distant than second cousins [20]. There is also greater ethnic diversity among the Omani population as a result of immigration from mainly South Asia over the past five centuries. Mixed marriages between people of Arab and Indian and mixed ethnicities have taken place as a matter of routine for centuries, thereby diluting the pool of abnormal alleles and consequently reducing the incidence of inherited/ genetic disorders.
Examination of Table 4 reveals patterns in relation to the most commonly detected disorders in the different regions and countries described. If VLCAD is excluded from the analysis for the time being (the special case of VLCAD is discussed below), the most common inherited metabolic/ endocrine disorders in Saudi Arabia (in all provinces investigated) are the inherited endocrine disorders CH and CAH. The most common inherited metabolic disorders in Central and Western Provinces of Saudi Arabia are propionic acidemia (PA) and PKU, while in Eastern and Jawf Provinces they are PKU, galactosemia (GALT) and 3-Methylcrotonyl-CoA Carboxylase Deficiency (3- MCC). These data therefore illustrate another interesting difference between Central/Western provinces of Saudi Arabia on the one hand and eastern/northern provinces on the other hand in that PA appear to be relatively common in Central/Western Saudi Arabia and relatively uncommon in Eastern/Northern Saudi Arabia. Furthermore, 3-MCC is relatively common in Eastern Province and relatively uncommon in Central/Western provinces. Indeed, within Eastern Province cases of 3-MCC appear to be concentrated in Al Ahsa (13 of the 15 cases of 3-MCC in our study were from Al Ahsa, Table 5) and 3-MCC was the most frequently detected inherited metabolic/endocrine disorder in the Al Ahsa study, even more common than CH and CAH [7].
VLCAD was the most frequently detected disorder in our study covering Eastern and Jawf Provinces, even more common than each of CH and CAH. It was also the only disorder which was more commonly encountered in Jawf province than Eastern Province, and mostly in the Jawf towns of Tubarjal (near the Jordanian border) and Dumah Al Jandal. The only other study that reported VLCAD incidence was the one from King Abdul-Aziz Medical City, Riyadh, Saudi Arabia, which reported an incidence of VLCAD in Central/Western Provinces of 1:36867 compared to our much higher VLCAD incidence for Eastern/Jawf provinces of 1:3211. In terms of Jawf Province alone the incidence of VLCAD is 1:439. The incidence of VLCAD in Jawf Province in particular is very high, and must be one of, if not, the highest in the world. Comparison incidence figures for VLCAD in the USA are approximately 1:40 000 [21]. In Jawf VLCAD is mostly prevalent within the Alsherarat tribe which carries a high frequency of the abnormal gene for VLCAD [22]. The Alsherarat tribe is mostly localized to certain parts of Jawf Province, and this explains the very low incidence of the disorder in other parts of Saudi Arabia [22-25].
Conclusion and Recommendation
In order to reduce the incidence of these disorders that cause significant morbidity the rates of consanguinity must be significantly reduced. Pre-marital screening must continue and the introduction of high-school carrier testing for the most common genetic disorders should be considered. VLCAD incidence in Jawf is the highest in Saudi Arabia and one of the highest in the world. Further studies are required and planned to determine the frequency of the abnormal allele in the Alsherarat tribe and in the Saudi population as a whole.
Compliance with Ethical Statement
Screening of newborn babies for these 17 disorders is an obligatory requirement set by the Saudi MOH, without which a birth certificate cannot be issued to the parents. Therefore, as our study required analysis of data from MOH sanctioned routine procedures we were not required to obtain informed consent or ethics approval.
Author Contributions
Randa Alratrout: Study design, collection, analysis and interpretation of data; writing of the report.
Zekreat Alsadah: Collection, analysis and interpretation of data; writing of the report.
Naseem Ansari: Analysis and interpretation of data; writing of the report.
Footnotes
The laboratory conducting the tests is monitored regularly through QC checks by the CDC in Atlanta with satisfactory feedback reports.
Acknowledgement
The authors wish to thank: The General Directorate for Prevention of Genetic and Chronic Diseases, MOH, for administrative and financial support of this program; The administration and Section for Technical Affairs at the Dammam Regional Laboratory and Blood Bank; and the following technical staff at the laboratory – Nesreen Alsadah, Ibtesam Bahehzer, fatema Rashed, Hejer Alfarag, Abdallah Hawsawi, Ahmad Aldosari, and Mohamad Alosami.
We would like to thank each of Asmaa Alrusaies, Laila Mathkour, Raneem Al-ghamdi, Aya Aldayel, Hanin Bashaikh and Fadah Alanazi from the College of Medicine, King Saud University for their help in data collection.
References
- Rashed MS. Clinical applications of tandem mass spectrometry: Ten years of diagnosis and screening for inherited metabolic diseases. J Chromatogr B Biomed Sci Appl 2001; 758: 27-48.
- General Authority for Statistics. Indicators. Riyadh: The Authority 2014.
- Abdul Salam A, Elsegaey I, Khraif R, et al. Population distribution and household conditions in Saudi Arabia: Reflections from the 2010 census. Springer Plus 2014; 3: 530.
- Alfadhel M, Al Othaim A, Al Saif S, et al. Expanded newborn screening program in Saudi Arabia: Incidence of screened disorders. J Pediatr Child Health 2017; 53: 585-591.
- Alfadhel M, Benmeakel M, Hossain MA, et al. Thirteen year retrospective review of the spectrum of inborn errors of metabolism presenting in a tertiary center in Saudi Arabia. Orphanet J Rare Dis 2016; 11: 126.
- Moammar H, Cheriyan G. Incidence and patterns of inborn errors of metabolism in the eastern province of Saudi Arabia, 1983-2008. Ann Saudi Med 2010; 30: 271-277.
- Al Bu Ali WH, Balaha MH, Al Moghannum MS, et al. Risk factors and birth prevalence of birth defects and inborn errors of metabolism in Al Ahsa, Saudi Arabia. Pan Afr Med J 2011; 8: 14.
- Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatr 2000; 105: e10.
- Joshi SN, Hashim J, Venugopalan P. Pattern of inborn errors of metabolism in an Omani population of the Arabian Peninsula. Ann Trop Paediatr 2002; 22: 93-96.
- Troxler H, Kleinert P, Schmugge M, et al. Advances in hemoglobinopathy detection and identification. Adv Clin Chem 2012; 57: 1-28.
- Seo JY, Park HD, Kim JW, et al. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: A retrospective and prospective evaluation. J Perinat Med 2014; 42: 121-127.
- Wiley V, Carpenter K, Wilcken B. Newborn screening with tandem mass spectrometry: 12 months’ experience in NSW Australia. Acta Paediatr 1999; 88: 48-51.
- Marquardt G, Currier R, McHugh DM, et al. Enhanced interpretation of newborn screening results without analyte cut-off values. Genet. Med 2012; 14: 648-655.
- Lindner M, Abdoh G, Fang-Hoffmann J, et al. Implementation of extended neonatal screening and a metabolic unit in the State of Qatar: Developing and optimizing strategies in cooperation with the Neonatal Screening Center in Heidelberg. J Inherit Metab Dis 2007; 30: 522-529.
- Al Hosani H, Salah M, Osman HM, et al. Expanding the comprehensive national neonatal screening programme in the United Arab Emirates from 1995 to 2011. East Mediterr Health J 2014; 20: 17-23.
- El-Mouzan MI, Al-Salloum AA, Al-Herbish AS, et al. Regional variations in the prevalence of consanguinity in Saudi Arabia. Saudi Med J 2007; 28: 1881-1884.
- Warsy AS, Al-Jaser MH, Albdass A, et al. Is consanguinity prevalence decreasing in Saudis? A study in two generations. Afr Health Sci 2014; 14: 314-321.
- Al Husain M, Al Bunyan M. Consanguineous marriages in a Saudi population and the effect of inbreeding on prenatal and postnatal mortality. Ann Trop Paediatr 1997; 17: 155-160.
- Teitelbaum J. Sunni vs. Shiite in Saudi Arabia. Jerusalem Center for Public Affairs. 2011; 10.
- Rajab A, Patton MA. A study of consanguinity in the Sultanate of Oman. Ann Hum Biol 2000; 27: 321-326
- Leslie ND, Valencia CA, Strauss AW, et al. Very long-chain acyl-coenzyme A dehydrogenase deficiency. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993-2017.
- https://ar.wikipedia.org/wiki/Alsherarat
- El-Hazmi MA, Al-Swailem AR, Warsy AS, et al. Consanguinity among the Saudi Arabian population. J Med Genet 1995; 32: 623-626.
- Otlowski MF, Taylor SD, Barlow-Stewart KK. Genetic discrimination: Too few data. Eur J Hum Genet 2003; 11: 1-2.
- Al-Arrayed S, Hafadh N, Amin S, et al. Student screening for inherited blood disorders in Bahrain. East Mediterr Health J 2003; 9: 344-352.