Current Pediatric Research
International Journal of Pediatrics
Role of urinary intestinal fatty acid binding protein in prediction of necrotizing enterocolitis and its correlation with GutCheck score.
Ibrahim Saad Hassan Abu Saif, Walaa Ahmed Yousry Kabiel, Aya Abdelaziz Abdelkarim, Wafaa Osman Ahmed*
Department of Pediatrics, Ain Shams University, Cairo, Egypt
- Corresponding Author:
- Wafaa
Osman Ahmed
Pediatrics Department
Faculty of Medicine
Ain Shams University
Tel: 01225117993
E-mail: wafaaosman83@gmail.com
Accepted date:26th August, 2021
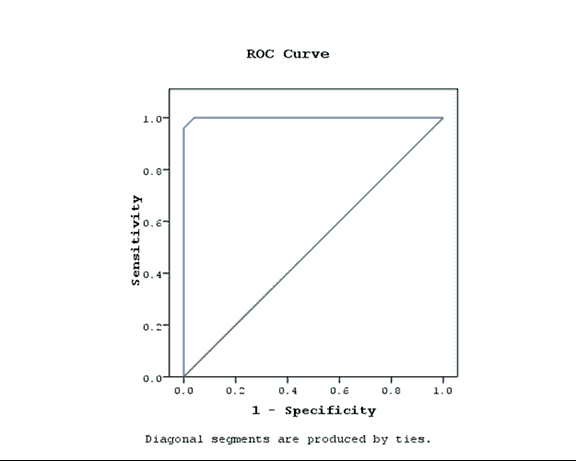
Background: Necrotizing Enterocolitis (NEC) has high neonatal morbidity and mortality, early prediction plays a role in prognosis; due to vague early symptoms and lack of specific biomarker. Objective: Value of urinary Intestinal Fatty acid Binding Protein for early diagnosis and prediction of NEC in correlation with GutCheckNEC score. Patients and Methods: A case control study included fifty preterm neonates over a period of one year, classified into two groups: 25 neonates with feeding intolerance prior NEC diagnosis with a gestational age 27-33 weeks regardless of birth weight matched with 25 healthy preterms, excluding neonates with previous abdominal surgery, congenital anomaly of the gastrointestinal tract or abdominal wall including gastroscisis, patients with known or suspected inflammatory disease, patients with acute kidney injury or parental refusal of enrollment. Results: GutCheckNEC score initially and after 14 days was significantly higher in cases compared to controls with P<0.01, blood transfusion, metabolic acidosis, out born status and low gestational age were associated with higher score initially (P<0.01,0.04,0.01 and 0.005 respectively), after 14 days blood transfusion, culture proved sepsis and metabolic acidosis had significantly higher score (P<0.04, 0.05 and 0.01),u-IFABP level tended to be higher in cases (P<0.001), Receiver operating characteristic curve (ROC curve) of NEC diagnosis using u-IFABP level showed cut-off >14 ng/mL with 96% sensitivity and 100% specificity. Conclusion: Both GutCheckNEC and u-IFABP have a good prognostic value in early diagnosis of NEC, however no correlation was found between score items and u-IFABP level.
Keywords
Necrotizing enterocolitis, Preterm neonates, NEC biomarkers, u-IFABP.
Introduction
Necrotizing Enterocoitis (NEC) is a gastrointestinal tract inflammatory disease of neonate’s especially preterm babies; it is an emergency for the clinician with high morbidity and mortality that mandates early diagnosis and proper treatment. In fact the etiology of NEC is multifactorial, however it is almost caused by antenatal hypoxia- ischemia and postnatal bacterial colonization and translocation in the immature gut, concluding that large Pataent Ductus Arteriosus (PDA), congenital heart disease, sepsis, prematurity and hemodynamic instability are predisposing factors for gut ischemia [1].
Early symptoms of NEC should be differentiated from sepsis, including temperature instability, hypotension, bradycardia or tachycardia as it could transform into life threatening intestinal necrosis and intestinal gangrene not rapidly treated by gastric decompression by ryle insertion, feeding withhold and possible antimicrobial treatment [2]. Mortality rate in NEC reaches more than 50% in neonates whose weight less than 1.5 kg, depending on the severity of affection and the percent is inversely related to weight [3]. Early diagnosis of NEC needs a fundamental laboratory indicator specially if combined with a clinical score to improve outcome, however it remains challenging especially before appearance of radiological signs.
One promising marker is the urinary Intestinal Fatty Acid Binding Protein (u-IFABP) that can diagnose early NEC with sufficient specificity and sensitivity and moreover could correlate with the severity of the disease and even the length of bowel resection in cases mandating surgical intervention. u-IFABP is a small cytoplasmic protein of the brush border of the enterocytes that play a role in the uptake and transport of polar lipids, so it is typically affected in case of damage of this border as occurs in NEC [4].
Fortunately, IFABP can be detected in serum and urine In conjunction with the lab, many scores were formulated to detect the risk for NEC and facilitate data interpretation among neonatologists, the most important is the GutCheckNEC tool which is composed of ten items, in turn each item is considered independent for occurrence NEC, they factors are gestational age, NICU-specific NEC rate, black or Hispanic race, out born, multiple infections, metabolic acidosis, packed Red Blood Cell (RBC) transfusion, and two risk reducers: human milk fed at both day 7 and 14, intake of probiotics. Clinical output of GutCheckNEC is translated into (B-range) for infants who developed surgical NEC and those who died and (C-range) for medical NEC [5].
Aim of the Work
Value of urinary Intestinal Fatty acid Binding Protein for early diagnosis and prediction of NEC is in correlation with GutCheckNEC score.
Patients and Methods
This case/control cross sectional study was conducted in the NICU, pediatric Hospital, Ain Shams University, in the period from May 2019 to May 2020 where 50 preterm babies (25 cases and 25 matching controls) were recruited. An informed consent was obtained. This study was approved from the local ethical committee of Ain Shams University. The study included (27 weeks or less-33 weeks) neonates, receiving oral feeding prior to NEC diagnosis. All neonate were monitored for feeding tolerance, where feeding intolerance is defined as increased gastric residuals exceeding 25% of the past fed, abdominal distension, unexplained bloody stools, or an X-ray showing bowel wall edema and/or emesis for at least 3 consecutive days leading to disruption of the feeding plan [5].
Neonates with previous abdominal surgery, gastrointestinal tract or abdominal wall anomalies and neonates with kidney impairment were excluded. All participants were recruited and a Full medical history after revision of the medical sheets for the feeding protocol regarding amount duration and type of feeding intake of probiotics, mechanical ventilation, antibiotics administrated, packed red blood cell transfusion, metabolic acidosis, inotropic medications for treatment of hypotension. Thorough general examination with documentation of vital signs (heart rate, blood pressure respiratory rate and temperature), activity and complexion and local examination for abdominal distension, tenderness, gardening, intestinal sounds and discoloration.
Blood samples were withdrawn for: Complete Blood Count (CBC) arterial blood gases for metabolic acidosis, defined as low pH associated with low serum bicarbonate (HCO3<17) but normal or near normal pCO2 (pH<7.30) or lactate >6.1 mmol/L [1]. Urine sampling for IFABP was performed for all patients with abdominal distension and feeding intolerance (bell stage 1) and for the matching 25 healthy controls, urine samples were collected either using sticky urine bag placed cautiously followed by urine evacuation into a sterile cup or directly through indwelling catheter, then were centrifuged and stored until analysis. During analysis, samples were brought to room temperature and tested using ELISA (we used human intestinal fatty acid binding protein ELISA kit), with procedure as recommended by the manufacturer’s recommendations:
Plain X-ray (gold standard): Done initially for all suspected NEC cases with feeding intolerance, then serial images were done for staging according to the Bell’s criteria. Findings were recorded including; dilated Intestinal loops, ileus, pneumatosis intestinalis, to confirm the diagnosis of NEC (gold standard). Only cases confirmed were retained in the study (25 neonates), while those with feeding intolerance that did not show radiological signs that favors the diagnosis of NEC were excluded. Pelvi-abdominal ultrasound: was done for all cases to assess bowel wall thickness, perfusion, echogenicity and peristalsis, portal venous gas, focal fluid collections and complex ascites. All participants were followed up for 14 days to confirm NEC through clinical examination, X-ray and pelvi-abdominal ultrasound.
Scoring by GutCheckNEC score was done with the appearance of feeding intolerance in cases, while for the controls; the score was performed in the matched postnatal date with the paired case simultaneously with collecting samples. The score items were collected from patient’s medical records then rescoring was done for preterm babies of group A that showed radiological signs of confirmed NEC, rescoring was done with each new risk factor, after 7 days and after 2 weeks. The score was done initially for group A (suspected NEC cases) and group B (healthy matching controls).
Results
This study is formulated of 50 preterm neonates divided into two groups: 25 cases and 25 controls free of NEC, 12(48%) males and 13 (52%) females in the case group and 14 (56%) males and 11(44%) females in the control group with mean gestational age 30 weeks ± 1.5 weeks for both groups with mean birth weight of 923 gm ± 113.7 gm among cases and 934 gm ± 115.5 gm among controls. 13(52%) cases were born in our hospital (current study setting) and 12(48%) were born outside while 15(60%) of the controls born inside and 10 (40%) outside with significantly high incidence of outbirth cases compared to controls and P-value 0.01. 80% versus 56% were delivered by cesarean section in both cases and controls respectively, While 20% versus 56% were born vaginally with high significant incidence of being cesarean born in cases compared to controls. Regarding breast feeding 7 (28%) of the cases were breast fed and 18 (72%) of them received formula feeding while 9 (36%) of controls received breast feeding and 16 (64%) received artificial formula with no significant difference in the incidence of breast feeding between cases and controls and P-value 0.54. As regards antibiotic administration 20%, 64% and 16% of the cases received single, double and triple antibiotics respectively, While 92%, 8% and 0% preterm controls received single, double and triple antibiotic regimen respectively. 16(54%) of the cases were mechanically ventilated while 11(44%) of the controls did not need respiratory support. Regarding blood culture proven sepsis, 32% versus 68% of the cases had negative and positive blood cultures respectively, while 84% versus 16% of controls had negative and positive blood culture respectively, NEC cases with positive blood culture were significantly higher than controls.
32(92%) cases experienced hypotension that needed inotropic medication (dopamine or doputamine), while only 11(44%) of the control group experienced hypotension, with significantly higher incidence of inotropic medication in cases compared to controls and P-value <0.001. metabolic acidosis occurred in 11(44%) cases, they had low pH (pH< 7.30) associated with low serum bicarbonate (HCO3<17) but normal or near normal pCO2 or lactate>6.1 mmol/L while 8(32%) of the group of controls experienced metabolic acidosis with no significant increase in the incidence of metabolic acidosis in cases compared to controls (P-value 0.3) packed red blood cell transfusion was received by 10 (40%) cases and 1(4%) of the controls.
Statistical methods
Statistical analysis was carried out using SPSS software (version 20.00). All data were presented as mean, standard error mean. P-value was calculated for each risk factor individually for the cases group in correlation with the control group and with the score (initially and after 14 days) and to detect the most significant factor affecting the score, P-value less than 0.05 was considered significant. Also, P-value was calculated for each demographic-clinical risk factor and risk factors included in the GutCheckNEC individually to detect the most significant risk factor affecting the marker level, Furthermore logistic regression analysis was done for the relationship between iFABP and NEC while controlling for potential confounders. To detect whether there was a significant difference between both scores the initial one and the one done 14 days after the onset of symptoms paired t test was conducted t<2 was insignificant also P-value was calculated.
Correlation coefficient (r) was done to confirm the linear correlation between the score/marker and each variable. To determine the sensitivity and specificity of the biomarker, receiver operating characteristics curves were assessed. The accuracy of the biomarker was determined by measuring the Area Under the Curve (AUC). The best cutoff points for the biomarkers were set at the maximum sum of sensitivity and specificity. The positive and negative likelihood ratios were calculated to measure likelihood of false positives and false negatives (Tables 1-8; Figure 1).
| No. 25 cases | No. 25 controls | P-value | ||||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Race | Black or hispanic | 0 | 0% | 0 | 0% | 0.7 |
| Otherwise | 25 | 100% | 25 | 100% | ||
| Gender | Male | 12 | 48% | 14 | 56% | 0.7 |
| Female | 13 | 52% | 11 | 44% | ||
| Birth place | Inborn | 13 | 52% | 15 | 60% | 0.5 |
| Outborn | 12 | 48% | 10 | 40% | ||
| Cesarean birth | Positive | 20 | 80% | 14 | 56% | 0.06 |
| Negative | 5 | 20% | 11 | 44% | ||
Table 1. Demographic data for the study groups. P-value<0.005 is significant; P-value<0.001 is highly significant.
| Number | % | Number | % | P-value | ||
|---|---|---|---|---|---|---|
| Breast feeding | Positive | 7 | 28% | 9 | 36% | 0.5 |
| Negative | 18 | 72% | 16 | 64% | ||
| Antibiotics | 2 antibiotics | 5 | 20% | 23 | 92% | <0.01 |
| 3 antibiotics | 16 | 64% | 2 | 8% | ||
| 4 antibiotics | 4 | 16% | 0 | 0% | ||
| Mechanical ventilation | Positive | 16 | 64% | 11 | 44% | 0.2 |
| Negative | 9 | 36% | 14 | 56% | ||
| Inotropes | Positive | 23 | 92% | 11 | 44% | <0.01 |
| Negative | 2 | 8% | 14 | 65% | ||
| Blood transfusion | Positive | 10 | 40% | 1 | 4% | 0.002 |
| Negative | 15 | 60% | 24 | 96% | ||
| Thrombocytopenia in CBC | Positive | 18 | 72% | 11 | 44% | 0.3 |
| Negative | 7 | 28% | 14 | 56% | ||
| Positive blood culture | Negative | 8 | 32% | 21 | 84% | <0.001 |
| Positive1 | 9 | 36% | 4 | 16% | ||
| Positive 2 | 8 | 32% | 0 | 0% | ||
| Metabolic acidosis | Positive | 11 | 44% | 8 | 32% | 0.3 |
| Negative | 14 | 56% | 68% | |||
Table 2. Comparison between the study groups regarding clinical data at diagnosis. P-value <0.005 is significant; P-value<0.001 is highly significant.
| Study group | Mean | SD | t | P-value | Sig. | |
|---|---|---|---|---|---|---|
| Marker | Cases | 48.32 | 36 | 6.32 | <0.001 | HS |
| Controls | 3.28 | 2.9 |
Table 3. Comparison of studied groups as regards marker.
| Score done initially | Score done after 14 days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | t | P-value | Mean | SD | t | P-value | ||
| Birth place | Inborn | 37.5 | 4.8 | 2.78 | 0.011 | 42 | 4.9 | 0.128 | 0.89 |
| Outborn | 32 | 5.2 | 41.8 | 4.8 | |||||
| Breast feeding | Negative | 35.3 | 5.9 | 0.558 | 0.582 | 42 | 5.3 | 0.198 | 0.485 |
| Positive | 33.9 | 5.2 | 41.6 | 3.5 | |||||
| Inotropic medication | Negative | 32.5 | 3.5 | -0.615 | 0.544 | 42 | 0 | 0.036 | 0.971 |
| Positive | 35.1 | 5.8 | 41.9 | 5 | |||||
| Blood transfusion | Negative | 31.5 | 3.9 | -5.571 | <0.001 | 40.4 | 4.7 | -2.02 | 0.049 |
| Positive | 40 | 3.5 | 44.1 | 4.1 | |||||
| Metabolic acidosis | Negative | 34.1 | 6.6 | -0.804 | 0.04 | 41.1 | 5.8 | -0.868 | 0.001 |
| Positive | 35.9 | 6.6 | 42.8 | 5.5 | |||||
| Gestational age | cases | 30.1 | 1.5 | (r) | 0 | (r) | 0.124 | ||
| controls | 30 | 1.6 | 0.722 | 0.316 | |||||
| Culture proved sepsis | cases | 35.6 | 5.8 | 1.061 | 0.07 | 42.1 | 4.5 | 3.186 | 0.005 |
| controls | 32.5 | 1.7 | 34.3 | 3.6 | |||||
Table 4. Correlation between each risk factor and GutCheckNEC score. P-value<0.005 is significant; P-value<0.001 is highly significant.
| Study group | X2 | P-value | Sig. | ||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||
| Initial score | Low risk | N. |
0 | 4 | 22.769 | <0.001 | HS |
% |
0.00% | 16.00% | |||||
| Moderate risk | N. |
7 | 19 | ||||
% |
28.00% | 76.00% | |||||
| High risk | N. |
11 | 2 | ||||
% |
44.00% | 8.00% | |||||
| Very high | N. |
7 | 0 | ||||
% |
28.00% | 0.00% | |||||
| Finalscore | low risk | N. |
0 | 3 | 26.262 | <0.001 | HS |
% |
0.00% | 12.00% | |||||
| Moderate risk | N. |
2 | 14 | ||||
% |
8.00% | 56.00% | |||||
| High risk | N. |
1 | 4 | ||||
% |
4.00% | 16.00% | |||||
| Very high | N. |
22 | 4 | ||||
% |
88.00% | 16.00% | |||||
Table 5. Comparison of initial and final score among studied groups.
| Initial score | Score done after 14 days | ||
|---|---|---|---|
| Weight | (r) | -0.361 | -0.211 |
| P-value | 0.076 | 0.311 | |
| Gestational age | (r) | -0.646 | -0.369 |
| P-value | 0 | 0.07 | |
| Postnatal age | (r) | 0.113 | -0.185 |
| P-value | 0.592 | 0.376 | |
| Antibiotics | (r) | 0.083 | -0.202 |
| P-value | 0.692 | 0.333 | |
| Gender | t | 0.88 | -0.712 |
| P-value | 0.388 | 0.484 | |
| Mechanical ventilation | t | -0.878 | 0.178 |
| P-value | 0.389 | 0.86 | |
Table 6. Correlation of initial and follow-up scores with other risk factors not included in the GutCheckNEC among cases.
| Risk stratification | Initial score | Score 14 d after | ||||||
|---|---|---|---|---|---|---|---|---|
| onset of symptoms | ||||||||
| cases | controls | cases | controls | |||||
| N | % | N | % | N | % | N | % | |
| Low risk <20 | 0 | 0% | 4 | 16% | 0 | 0% | 3 | 125 |
| Moderate risk 20-32 | 7 | 28% | 19 | 76% | 2 | 8% | 14 | 56% |
| High risk 33-36 | 11 | 44% | 2 | 8% | 1 | 4% | 4 | 16% |
| Very high risk >36 | 7 | 28% | 0 | 0% | 22 | 88% | 4 | 16% |
Table 7. Risk stratification according to GutCheckNEC for cases-controls.
| Area under curve | P-value | Asymptotic 95% confidence interval | |
|---|---|---|---|
| Upper bound | Lower bound | ||
| 0.999 | 0 | 0.996 | 1.003 |
Table 8. ROC Curve of the marker.
Discussion
Recently many tools have been reported to have high sensitivity and specificity in diagnosis of NEC in early stages so, this study was carried out to investigate the most sensitive and early predictor of NEC among two tools and to study the correlation between them; these tools were:
Urinary intestinal fatty acid binding protein (uIFABP) the biologic marker and GutCheckNEC risk index a scoring system formulated by Gephart et al. in 2014, the score is formulated after 3 phases of refinement to pick up the most relevant risk factors and still needs further research and refinement [1]. These factors are (gestational age, race, outborn, NICU NEC rate: Unknown in our study setting and assigned 16 for all enrolled neonates as stated by the author, exclusive human milk feeding as a risk reducer and defined as human milk fed at both day 7 and day 14 of life, Probiotics as a risk reducer (was not available in our NICU), culture-proved infections after the third day of life, Packed Red Blood Cell transfusion, Hypotension treated with Inotropic medication and Metabolic Acidosis). We correlated each item that represents a possible risk factor with the score initially and after 14 days GutCheckNEC initial score was significantly high with low gestational age (P=0.00) CI(-0.64). Similarly Gephart reported a significantly higher incidence of NEC in preterm babies with low gestational age with P-value<0.001 for G. A 28-33 weeks, similar results reported by Niño who concluded that low gestational age is the most important independent risk factor for NEC development and mortality with P-value <0.01 that could be attributed to increased incidence of blood transfusion, sepsis, antibiotic intake and umbilical catheterization with low gestational age [1,6]. The score also was higher in babies with culture proved sepsis after 14 days with P-value 0.005. Similarly, Manas Ranjan who conducted a prospective study of 45 neonates with necrotizing enterocolitis in India and noticed sepsis in 88.8% of NEC cases and concluded that sepsis is a highly significant risk factor for NEC prognosis with P-value 0.001. Same results were also confirmed by Lee et al. who detected sepsis in one-thirds of neonates with NEC [7,8].
Metabolic acidosis had a highly significant effect on the score after 14 days with P-value 0.01 (sign of severe infection or sepsis), similarly Tepas who studied 7 Components of Metabolic Derangement (CMD), consisting of positive blood culture, acidosis, bandemia, thrombocytopenia, hyponatremia, hypotension, or neutropenia and concluded that preterm babies with matabolic derangement had higher incidence of NEC mortality and morbidity with P-value 0.02 [9]. Preterm babies who received packed red blood cells had significantly higher score with P-value <0.001 initially and 0.049 after 2 weeks. Similarly Wan-Huen who conducted a case-control study in New York concluded that NEC is strongly associated with blood transfusion with CI (1.46, 6.05) P=0.003 [10]. Same results were confirmed by Christensen and El-Dib, which could be justified by many theories including: reperfusion injury due to change in the viscosity of blood following PRBC transfusions, alteration and disruption of gut perfusion leading to mesenteric ischemia in post-prandial blood transfusion or Immunological or non-immunological reaction elicited by blood transfusion leading to NEC [11-15].
Preterm babies born outside our study setting had a significantly higher score initially (p=0.01) while after 14 days P-value 0.89. Similarly Yee who conducted a population-based cohort of 16,669 infants with Gestational Age (GA) <33 weeks, admitted to 25 NICUs and reported high incidence of NEC in outborn babies(P=0.001)due to multiple transmission that may have increased risk of exposure to stress and infection. Breastfeeding had no significant effect on the score neither initially nor after 14 days(P=0.5 and 0.48 respectively), opposing to our results Gephart considered breast feeding a risk reducer with item weight-3 this can be explained by the non-clear feeding protocol, mixed feeding (breast milk and formula mik) and introduction of high osmolar formula which increases NEC risk [16].
In our study hemodynamic instability and hypotension requiring inotropic treatment had no significant effect on the score neither initially nor after 14 days with P-values 0.5 and 0.9 respectively in contrast to Gephart who reported that hypotension requiring inotropic treatment was associated with an increased risk of NEC (P=0.001) [1]. However many recent studies suggested that dopamine infusion is not associated with any increase in cardiac output or superior vena cava flow or superior mesenteric artery blood flow [17,18].
We studied the overall score significance and found that both initial and follow up score were significantly higher in cases compared to controls, the score done 14 days after onset of symptoms was significantly high compared to the initial score, that was confirmed by the paired t test that showed paired t=-6.746 and P-value<0.001 which is highly significant. We stratified NEC cases according to the Gephart model and found that initially 16%, 76%, 8% and 0% had low, moderate, high and very high risk respectively while after 14 days the ratios were 12%, 56%, 16% and 16% low, moderate, high and very high risk this was confirmed by the paired t test that showed paired t=-6.746 and P-value<0.001 which is highly significant.
We studied other clinical factors that could be associated with high incidence of NEC and not included in the Gephart’s model male gender was not associated with significantly higher score compared to female neither initially nor after 14 days with P-values >0.005 Gephart reported same incidence of NEC in male preterm babies compared to females [1]. On the contrary ManasRanjan reported that female gender predominance with female to male ratio 1.13:1 [7].
Regarding mode of delivery there was no statistical difference in the GutCheckNEC score between preterm babies delivered by cesarean section compared to those delivered vaginally neither initially nor after 14 days with P-values >0.005. Similarly, Gephart excluded cesarean birth as a risk reducer from their model due to multiple maternal risk factors associated with cesarean section and could be potential confounders [1]. On the contrary Samuels et al. reported that birth by cesarean section was associated with a decreased risk of NEC as it decreases stress exposure during labor [19].
Regarding mechanical ventilation the GutCheckNEC score of preterm babies who needed assisted ventilation had no significant statistic difference compared to those who did not need assisted ventilation with P-values 0.3 and 0.86 initially and 14 days after the onset of symptoms respectively this comes in agreement with Gephart who excluded mechanical ventilation as an independent risk factor due to multiple confounders (respiratory distress) [1]. On the contrary Carter reported a higher incidence of NEC among preterm babies that needed assisted ventilation [20]. We studied the factors affecting final bell stage of both case-control pairs and concluded that it was not affected by weight, gestational age, post natal age, antibiotics or marker level with P-values: 0.883, 0.680, 0.118, 0.088, 0.233 respectively, Furthermore, it was not affected by risk stratification according to GutCheckNEC
Regarding urinary intestinal fatty acid binding protein (u-IFABP) many studies have reported IFABP as early sensitive tool for early prediction of NEC [21]. So we measured the urinary level when early symptoms developed and used it as a marker for early detection of NEC, NEC cases tended to have a higher level of the marker, mean level in suspected NEC cases 48.32 ± 35.6 ng/ml (range 18-160) with 16 fold increase in cases than controls that had mean level of 3.28 ± 2.9 ng/ml (range 2-10). That was roughly the same range detected by Gregory who conducted a cohort study including 70 preterm babies born less than 29 weeks gestation admitted to the neonatal intensive care unit at Brigham and reported mean level 15 ng/ml (range 6-39) among cases and 3 ng/ml among controls (range 2-30) [22].
Correlating marker level with each risk factor in the GutCheckNEC score and other studied risk factors we found that male gender, cesarean birth, antibiotic administration, Bell staging and items of the GutCheckNEC score (gestational age, out birth, race, breast feeding, hemodynamic instability requiring inotropic medications, positive blood culture, blood transfusion, metabolic acidosis) showed no significant effect on the marker level, Further more logistic regression analysis showed that regression coefficient of the marker was only affected by positive blood culture, blood transfusion and inotropic medications with P-values 0.037, 0.141 and 0.139 respectively, while other factors had minimal impact.
Similarly, Gregory [22] reported that mode of delivery, human milk exposure, growth restriction, antenatal steroids, Bell stage and 5-minute Apgar score had minimal impact on the regression coefficient (iFABP OR 4.73 (2.44, 9.15) in the 7-day analysis). Though the GutCheckNEC score was a good indicator of early stages of NEC in preterm infants the marker level did not show significant correlation (increase-decrease) with cases when stratified into low-moderate-high-very high risk according to the score. Our study concluded that we can early diagnose NEC through ROC curve with cut off: >14 ng/ml with 96% sensitivity and 100% specificity and the Area Under Receiver operating characteristics curve (AUC) 0.99. Similarly Gregory reported a cut-off value >13.3 ng/mL with 60% sensitivity and 78% specificity (c-statistic=0.74) within 7 days of NEC [22].
Conclusion
Both GutCheckNEC and u-IFABP has a good prognostic value in early diagnosis of NEC. However no correlation was found between score items and u-IFABP level.
References
- Gephart SM, Spitzer AR, Effken JA, et al. Discrimination of GutCheck (NEC): A clinical risk index for necrotizing enterocolitis. J Perinatol 2014; 34(6): 468-75.
- Gephart SM, Hanson C, Wetzel CM, et al. NEC-zero recommendations from scoping review of evidence to prevent and foster timely recognition of necrotizing enterocolitis. J Matern Health Neonatol Perinatol 2017; 3: 23.
- Wiswell TE, Robertson CF, Jones TA, et al. Necrotizing enterocolitis in full-term infants. A case-control study. Am J Dis Child 1988; 142(5): 532-5.
- Schurink M, Scholten IGH, Kooi EMW, et al. Intestinal fatty acid-binding protein in neonates with imminent necrotizing enterocolitis. J Neonatology 2014; 106(1): 49-54.
- Thomas S, Nesargi S, Roshan P, et al. Gastric residual volumes versus abdominal girth measurement in assessment of feed tolerance in preterm neonates. Adv Neonatal Care 2018; 18(4): E13-9.
- Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016; 13(10): 590-600.
- Sahoo MR, Nagasree P, Swetha L, et al. Risk factors for necrotizing enterocolitis in neonates: A prospective study. Int J Contemp Pediatr 2019; 6(4):1598-1602.
- Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol 2003; 8(6): 449-59.
- Tepas JJ, Sharma R, Hudak ML, et al. Coming full circle: An evidence-based definition of the timing and type of surgical management of very low-birth-weight (<1000 g) infants with signs of acute intestinal perforation. J Pediatr Surg 2006; 41(2): 418-22.
- Wan-Huen P, Shapiro DM, Bateman D, et al. Packed red blood cell transfusion is an independent risk factor for necrotizing enterocolitis in premature infants. J Perinatol 2013; 33(10): 786-90.
- Christensen RD. Association between red blood cell transfusions and necrotizing enterocolitis. J Pediatr 2011; 158(3): 349-50.
- El-Dib M, Narang S, Lee E, et al. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol 2011; 31(3): 183-7.
- Nelle M, Hocker C, Zilow EP, et al. Effects of red cell transfusion on cardiac output and blood flow velocites in cerebral and gastrointestinal arteries in premature infants. Arch Dis Child Fetal Neonatal Ed 1994; 71(1): 45-8.
- Gretchen AK, Baker R, Yanowitz TB. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol 2009; 26(2): 99-105.
- Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg 2009; 108(3): 759-69.
- Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. J Pediatrics 2012; 129(2): 298-304.
- Hentschel R, Hensel D, Brune T, et al. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biol Neonate 1995; 68(5): 318-24.
- Pearson RJ, Barrington KJ, Jirsch DW, et al. Dopaminergic receptor-mediated effects in the mesenteric vasculature and renal vasculature of the chronically instrumented newborn piglet. Crit Care Med 1996; 24(10): 1706-12.
- Samuels N, Graaf RAV, de Jonge RCJ, et al. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr 2017; 17(1): 105.
- Carter BM, Holditch-Davis D. Risk factors for necrotizing enterocolitis in preterm infants: How race, gender, and health status contribute. Adv Neonatal Care 2008; 8(5): 285-90.
- Coufal S, Kokesova A, Tlaskalova-Hogenova H, et al. Urinary intestinal fatty acid-binding protein can distinguish necrotizing enterocolitis from sepsis in early stage of the disease. J Immunol Res 2016; 2016: 1-8(5727312).
- Gregory K, Winston AB, Yamamoto HS, et al. Urinary intestinal fatty acid binding protein predicts necrotizing enterocolitis J Pediatr 2014; 164(6): 1486-8.