Current Pediatric Research
International Journal of Pediatrics
Profile of expert MTB/RIF in the diagnosis of children with suspected tuberculosis in tertiary hospital in Surabaya, Indonesia.
Adwina Nurlita Kusuma Wardhani*,Retno Asih Setyoningrum
Department of Child Health, Faculty of Medicine Airlangga University, Surabaya, Indonesia
- Corresponding Author:
- Dr. Adwina Nurlita Kusuma Wardhani
Department of Child Health
Faculty of Medicine Airlangga University
Surabaya
Indonesia
E-mail: adwinanurlita@gmail.com
Received: 08-Dec-2021, Manuscript No. AAJCP-22-51155;
Editor assigned: 13-Dec-2021, PreQC No. AAJCP-22-51155 (PQ);
Reviewed: 27-Dec-2021, QC No. AAJCP-22-51155;
Revised: 08-Feb-2022, Manuscript No. AAJCP-22-51155 (R);
Published: 15-Feb-2022, 10.35841/0971-9032.26.5.1422-1426.
Citation: Wardhani ANK, Setyoningrum RA. Profile of expert MTB/RIF in the diagnosis of children with suspected tuberculosis in tertiary hospital in Surabaya, Indonesia. Curr Pediatr Res. 2022;26(5): 1422-1426.
Background: The accurate diagnosis of childhood tuberculosis remains a major challenge. Molecular diagnostic tests with expert MTB/RIF that are more rapid and or less expensive compared to the conventional culture techniques are used for diagnosis and drug-resistance testing. This study aims to describe the characteristics of children with suspected tuberculosis tested expert MTB/RIF in a tertiary hospital in Surabaya, Indonesia. Methods: A descriptive study was conducted on children with suspected tuberculosis in an academic teaching hospital in Surabaya, Indonesia between January 2016 and December 2020. Clinical materials were submitted to the laboratory for examination including sputum, pleural fluid, and gastric aspirate and expert MTB/RIF test was performed, with the outcomes described as MTB Detected, MTB Not Detected, rifampicin-sensitive, and rifampicin-resistant. Results: Among 887 subjects during five years study period, 253 (28.5%) were sputum samples, with others from gastric aspirate, cerebrospinal fluids, faces, material from abscesses, and other biopsy specimens or resected tissue. The total number of MTB Detected was 155 (17.5%) with the majority collected from 109 (70.3%) sputum samples, and the highest number of MTB Detected was found in age 10-18 years old 105/155 (67.7%). Drug sensitivity test showed 13 (8. 3%) rifampicin resistance with all samples were sputum, while 142 (91.6%) subjects showed no rifampicin resistance. Conclusion: Largest sample collections of children tested expert MTB/RIF were sputum, showing a high number of MTB positivity, with the majority aged 10-18 years old. Drug sensitivity test showed rifampicin-resistant results came from sputum samples
Keywords
Tuberculosis, Children, Expert MTB/RIF, Profile.
Introduction
Tuberculosis (TB) is one of the major causes of paediatric mortality, with half a million cases every year in the world [1]. The number of Indonesian paediatric TB cases fluctuates each year, indicating the need to evaluate the paediatric TB diagnosis’ quality in Indonesia [2].
World Health Organization recommends expert MTB/RIF which could detect Mycobacterium Tuberculosis (MTB) and rifampicin-resistance less than 100 minutes [3]. A study found that it could detect 90.3% MTB. Another study found that children's sputum provides high accuracy. This study aims to describe the profile of suspected paediatric TB tested with expert MTB/RIF in a tertiary hospital in Surabaya, Indonesia.
Methods and Materials
Patients
A retrospective descriptive study was performed in an academic teaching hospital in Surabaya, Indonesia. The accessible population was all children with suspected TB in the same hospital who had undergone rapid molecular test expert MTB/RIF for the period of study. Children aged 0-18 years who had incomplete medical record data were excluded from this study. A total of 887 children were recruited in this study. Ethical approval for this study was obtained from The Ethics Committee of the Faculty of Medicine, Universities Airlangga Dr. Soetomo Academic General Hospital with number 1037/KEPK/III/2019 [4].
Data collection procedures
This study uses secondary data from the results of rapid molecular test expert MTB/RIF in the period of January 2016 December 2020. Data were collected from the medical records in the Multidrug Resistance Tuberculosis outpatient clinic of the hospital. The data were based on a variety of specimens submitted to the laboratory for examination, including sputum, pleural fluid, and gastric aspirate from the hospital and outside the hospital. The diagnostic test used expert MTB/RIF, with the outcomes described as MTB detected, MTB not detected, rifampicin-sensitive and rifampicin-resistant.
Measures
Total sampling was conducted for this study. After population recruitment, it is then classified into 4 groups of variables: Demographic data, sample characteristics, expert MTB/RIF results, and rifampicin resistance [5].
Statistical analysis
The data were analysed using a descriptive statistical test with version 22.0: SPSS Software, IBM, and Chicago, IL, USA. Statistical analysis was based on the calculation of frequency distribution.
Results
A total of 887 data were obtained with the characteristic based on clinical sample presented. Table 1 shows that, from 2016 until 2020, most samples collected for TB diagnosis are sputum (28.5%) followed by mucus (24.4%) and saliva (16.6%). Nevertheless, samples from stools and biopsy tissues were increasing in the last two years (Table 1).
Table 1. Clinical sample characteristics.
| Sample | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|
| Sputum | 37 (58.7%) | 38 (43.7%) | 39 (31.2%) | 72 (19.6%) | 67 (27.5%) | 253 (28.5%) |
| Mucus | 14 (22.2%) | 25 (28.7%) | 48 (38.4%) | 92 (25%) | 37 (15.2%) | 216 (24.4%) |
| Saliva | 9 (14.3%) | 20 (23%) | 19 (15.2%) | 68 (18.5%) | 31 (12.7%) | 147 (16.6%) |
| Gastric aspirate | 0 (0%) | 2 (2.3%) | 14 (11.2%) | 37 (10.1%) | 58 (23.8%) | 111 (12.5%) |
| CSF* | 1 (1.6%) | 0 (0%) | 2 (1.6%) | 37 (10.1%) | 13 (5.3%) | 53 (6%) |
| Stool | 0 (0%) | 0 (0%) | 0 (0%) | 31 (8.4%) | 13 (5.3%) | 44 (5%) |
| Biopsy | 0 (0%) | 1 (1.1%) | 3 (2.4%) | 23 (6.2%) | 19 (7.8%) | 46 (5.2%) |
| Others | 2 (3.2%) | 1 (1.1%) | 0 (0%) | 8 (2.2%) | 6 (2.5%) | 17 (1.9%) |
| Total | 63 | 87 | 125 | 368 | 244 | 887 |
*CSF refers to cerebrospinal fluid
Among 887 participants, MTB detected and MTB not detected were 155 patients and 732 patients respectively [7]. More than half of participants who had MTB detected are found in the 10-18 years age group, classified into rifampicin-sensitive (66.2%) and rifampicin-resistant (84.6%). Despite that, the highest number of MTB Not Detected is found in this age group as well (Table 2).
Table 2. Expert MTB/RIF result based on age.
| Age (Year) | Expert MTB*/RIF† Result | Total | ||
|---|---|---|---|---|
| MTB detected | MTB detected | MTB Not Detected | ||
| RIF Sensitive | RIF Resistant | |||
| 0-1 | 20 | 0 | 239 | 259 |
| (14.1%) | 0% | (32.6%) | ||
| 01-Apr | 13 | 1 | 112 | 126 |
| (9.1%) | (7.7%) | (15.3%) | ||
| 05-Sep | 15 | 1 | 128 | 144 |
| (10.6%) | (7.7%) | (17.5%) | ||
| Oct-18 | 94 | 11 | 253 | 358 |
| (66.2%) | (84.6%) | (34.6%) | ||
| Total | 142 | 13 | 732 | 887 |
*MTB refers to Mycobacterium tuberculosis
RIF refers to rifampicin
The results of expert MTB/RIF test based on age and clinical specimens. It is found that MTB Detected is predominant in the 10-18 years group (n=101). Primarily, the clinical samples were from sputum (n=84) (Table 3).
Table 3.Expert MTB/RIF results based on age and clinical specimen.
| Age (Year) | Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sputum | Gastric aspirate | CSF* | Stool | Biopsy | ||||||
| Detected | Not | Detected | Not | Detected | Not | Detected | Not | Detected | Not | |
| 0-1 | 9 | 27 | 8 | 61 | 2 | 25 | 1 | 8 | 0 | 5 |
| 44652 | 7 | 24 | 4 | 14 | 0 | 3 | 1 | 11 | 1 | 6 |
| 44809 | 9 | 26 | 2 | 8 | 0 | 7 | 0 | 6 | 3 | 3 |
| 43374 | 84 | 67 | 3 | 11 | 5 | 11 | 2 | 15 | 7 | 21 |
| Total | 109 | 144 | 17 | 94 | 7 | 46 | 4 | 40 | 11 | 35 |
*CSF refers to cerebrospinal fluid
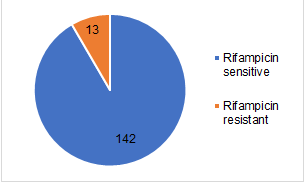
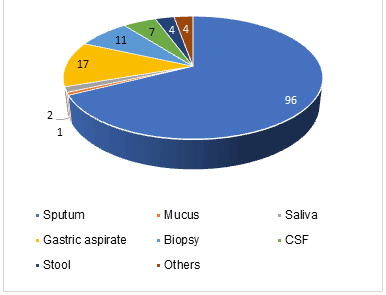
From 155 participants with MTB Detected results, almost all of the subjects (142 (91.6%)) have rifampicin-sensitive and only a few subjects (13 (8.4%)) have a rifampicin-resistant type [8, 9]. All samples from 13 patients, who had rifampicin-resistant MTB, were from sputum. Thus, concurrent with rifampicin-sensitive group examination, the majority of the results were from sputum (96 (67.6%)) followed by gastric aspirate (17 (11.97%)) and biopsy tissue (11 (7.7%)) Blue represents rifampicin-sensitive. Orange represents rifampicin-resistant. Figure 2 represents the results of sample collection in the rifampicin-sensitive group (Figures 1 and 2).
Discussion
This study occurred by dint of early diagnosis obstacle especially in suspected paediatric TB patients which is crucial for TB management. Some conventional TB diagnosis instruments already existed, such as clinical approach with TB score, acid-fast bacillus examination, and MTB culture [10]. All of these had a weakness, including MTB culture, the gold standard for TB diagnosis. It takes six to eight weeks of the diagnostic process which might delay patients’ initial treatment. Sputum examination with expert MTB/RIF, a rapid molecular test using polymerase chain reaction DNA, provides faster and more sensitive results than the other conventional instruments [11].
Our findings showed that among 887 children with suspected TB who had been tested with expert MTB/RIF, there were 253 (28.5%) specimens from sputum. Concurrent with most samples come from the sputum of TB patients. The study also showed that expert MTB/RIF has high sensitivity (98%-100%) on positive BTA and culture samples, although sensitivity on negative BTA samples is found to be only 43%-70% [12]. Conducted a similar study in children and found that expert MTB/RIF sensitivity increased 27.8% with 98.8% specificity.
TB mainly occurs in the lung, yet extra pulmonary TB is also found in numerous cases. Revealed that samples from sputum have higher sensitivity and specificity than samples from gastric aspirate in diagnosis TB with expert MTB/RIF. The sensitivity was 26.7% and 22.6%, while specificity was 100% and 99.6% from sputum induction and gastric aspirate samples respectively. Yet, from 12 researches, revealed that the sensitivity of expert MTB/RIF on gastric aspirate samples from TB pulmonary patients was 83.8% with 98.1% specificity. Simultaneously with a. expert MTB/RIF examination on extra pulmonary samples (urine, biopsy tissue, pleural aspirate, cerebrospinal fluid, and stools) from TB patients have 77.3% sensitivity and 98.2% specificity in general. Exclusively, biopsy tissue has 69.0% sensitivity, and urine and stools have 100% sensitivity [13].
A study in South Africa using expert MTB/RIF in extra pulmonary TB patients with the sample from stools. They found that sensitivity and specificity were unexpectedly high with 31.9% (95%CI 21, 84-44, 50) for sensitivity and 99.7% (95% CI 98, 2-100) for specificity. In China revealed that extra pulmonary TB examination from bronchoalveolar lavage has 72.9% and 98.7% for sensitivity and specificity respectively. However, expert MTB/RIF examination on several specimens for one patient reportedly does not increase the case detection rate [14]. Hence, in daily clinical practice, expert MTB/RIF test using two or more types of samples have to be considered regarding its risk and benefit since there are other diagnostic examination methods.
The results from our study showed that among 155 expert MTB/RIF detected patients, 109 (70.3%) comes from sputum specimen, predominantly in the 10-18 years age group (n=101). Teenagers tend to have a higher risk to be infected by TB, especially post-menarche female patients. This is due to children under ten years old generally having paucibacillary TB type, leading to a low MTB detected. Primary infection during teenage years is related to adult-type TB disease development, resulting in more sputum accumulation and usually gives positive examination results. This type could be developed by primary infection, endogen reactivation, and hexogen reactivation. Therefore, the possibility of positive results from expert MTB/RIF is increased. Revealed that this type occurs in children above ten years post-primary infection.
The drug sensitivity test showed 13 (8.3%) rifampicin resistance with all samples were sputum, while 142 (91.6%) subjects showed no rifampicin resistance. Concurrent with our findings, revealed that in paediatric TB patients, 7.9% rifampicin-resistant MTB were positive [15]. Yet another study, which was conducted in Indonesia, showed that among children with suspected pulmonary TB who tested positive expert MTB/RIF, 100% were rifampicin sensitive Positive results of MTB rifampicin resistance indicate that MTB bacteria possibly have a high resistance to rifampicin.
Since this study was retrospective research merely using secondary data, limitation in our study includes not all samples tested by culture MTB and incomplete medical record. Therefore, comprehensive analysis of TB contact history, total TST, anthropometry status, thorax x-ray result, anatomical location, medical history of TB, also HIV infection status were difficult to be done.
Conclusion
The largest sample collections of children who tested expert MTB/RIF were sputum, with a high number of MTB positivity and majority from 10-18 years old age group. Drug sensitivity test showed all rifampicin-resistant results came from sputum samples.
Based on World Health Organization estimation in 2020, a 25% decrease in TB case detection over 3 months will result in a 13% increase in the number of deaths due to TB; delaying the progress of successful TB programs in the last 5 years. It is estimated that there will be an additional 1.4 million deaths in TB patients due to the consequences of COVID-19 in 2020-2025.
Based on these results, further research was recommended to determine and compare diagnostic value between the rapid molecular test using expert MTB/RIF, the microscopic examination of Acid-Fast Bacilli, and the MTB culture using a cohort method; also identify more complete data related to positive MTB/RIF result. This is to succeed in the national target of reducing TB incidence and mortality by increasing the number of children diagnosed and treated, as well as investigation of household contacts to conduct TB prevention therapy at the right time.
Conflict of Interest
This research has no conflict of interest.
References
- Sharma SK, Kohli M, Yadav RN, et al. Evaluating the diagnostic accuracy of expert MTB/RIF assay in pulmonary tuberculosis. PloS One. 2015;10:e0141011.
[Crossref] [Google Scholar] [Pubmed]
- Nicol MP, Workman L, Isaacs W, et al. Accuracy of the expert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis. 2011;11:819-824.
[Crossref] [Google Scholar] [Pubmed]
- Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. The lancet. 2011;377:1495-1505.
[Crossref] [Google Scholar] [Pubmed]
- Agustina B, Kartasasmita C, Hilmanto D. Comparison of gene pert mtb to mycobacterium tuberculosis culture in children with tuberculosis. Paediatr Indones. 2019;59:113-118.
- Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the gene pert mtb/rif assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138-4141.
[Crossref] [Google Scholar] [Pubmed]
- Walusimbi S, Bwanga F, De Costa A, et al. Meta-analysis to compare the accuracy of gene pert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis. 2013;13:1-3.
[Crossref] [Google Scholar] [Pubmed]
- Bunyasi EW, Tameris M, Geldenhuys H, et al. Evaluation of expert MTB/RIF assay in induced sputum and gastric lavage samples from young children with suspected tuberculosis from the MVA85A TB vaccine trial. PloS one. 2015;10:e0141623.
[Crossref] [Google Scholar] [Pubmed]
- Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis and treatment of tuberculosis in HIV co-infected patients. Indian J Med Res. 2011;134:850.
[Crossref] [Google Scholar] [Pubmed]
- Hillemann D, Rüsch-Gerdes S, Boehme C, et al. Rapid molecular detection of extra pulmonary tuberculosis by the automated gene pert MTB/RIF system. J Clin Microbiol. 2011; 49:1202-1205.
[Crossref] [Google Scholar] [Pubmed]
- Walters E, van der Zalm MM, Palmer M, et al. Expert MTB/RIF on stool is useful for the rapid diagnosis of tuberculosis in young children with severe pulmonary disease. Pediatr Infect Dis J. 2017; 36:837.
[Crossref] [Google Scholar] [Pubmed]
- Lu Y, Zhu Y, Shen N, et al. Evaluating the diagnostic accuracy of the expert MTB/RIF assay on bronchoalveolar lavage fluid: a retrospective study. Int J Infect Dis. 2018; 71: 14-9.
[Crossref] [Google Scholar] [Pubmed]
- Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era (State of the Art). Int J Tuberc Lung Dis. 2004; 8:392-402.
[Google Scholar] [Pubmed]
- Sekadde MP, Wobudeya E, Joloba ML, et al. Evaluation of the expert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: a cross-sectional diagnostic study. BMC Infect Dis. 2013;13:1-8.
[Crossref] [Google Scholar] [Pubmed]
- Arega B, Menbere F, Getachew Y. Prevalence of rifampicin resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19(1):1-5.
[Crossref] [Google Scholar] [Pubmed]
- Rarome BB, Aisah N, Setyoningrum RA, et al. Gene pert MTB/RIF and mycobacterium tuberculosis Sputum culture in establishing the diagnosis of pulmonary tuberculosis and rifampicin resistance in suspected childhood pulmonary tuberculosis in soetomo hospital. Indonesian Journal of Tropical and Infectious Disease. 2020;8:152-60.