Current Pediatric Research
International Journal of Pediatrics
Post-therapy neuronal maturation in an adult SHH medulloblastoma
1Department of Medical Oncology, Bellaria-Maggiore Hospitals, Azienda USL, IRCCS Institute of Neurological Sciences, Bologna, Italy.
2Department of Neuropathology, University of Heidelberg, Heidelberg, Germany; Clinical Cooperation Unit Neuropathology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
3Department of Pathology, Bellaria-Maggiore Hospitals, Azienda USL, IRCCS Institute of Neurological Sciences, Bologna, Italy.
4Department of Radiological, Oncological and Anatomo-Pathological Sciences, Sapienza University, Rome, Italy.
5IRCCS Neuromed, Pozzilli (Is), Italy.
6Section of Pathological Anatomy, Polytechnic University of the Marche Region, School of Medicine, United Hospitals, Ancona, Italy.
7Department of Neuroradiology, Bellaria-Maggiore Hospitals, Azienda USL, IRCCS Institute of Neurological Sciences, Bologna, Italy.
8Department of Neurological Sciences, Section of neurosurgery, United Hospitals, Ancona, Italy.
9Department of Radiotherapy, Bellaria-Maggiore Hospitals, Azienda USL, IRCCS Institute of Neurological Sciences, Bologna, Italy.
10Department of Medicine (DIMES) Anatomic Pathology-Molecular Diagnostic Unit AUSL of Bologna, University of Bologna School of Medicine, Bologna, Italy.
11Department of Pharmacy and Biotechnology (FaBiT), Molecular Pathology Unit AUSL di Bologna, Bellaria Hospital, University of Bologna, Bologna, Italy.
12Department of Biomedical and Neuromotor Sciences, Section of Anatomic Pathology, University of Bologna, Bellaria Hospital, Bologna, Italy.
- Corresponding Author:
- Alba A Brandes
Department of Medical Oncology
Azienda USL-IRCCS Institute of Neurological Sciences
Via Altura 3, 40139 Bologna, Italy
Tel: +39 (0)51 6225697
Fax: +39 (0)51 6225057
E-mail: alba.brandes@yahoo.it
Accepted date: August 30, 2017
Medulloblastoma is an extremely rare disease in adults. After surgery, the mainstay of treatment is based on chemotherapy and radiotherapy, that provide 5 year suvival rates of 50-80% according to risk factors. Post-therapy nauronal maturation in medulloblastoma is a rare event that has been described only in pediatric age. Here we report the case of neuronal maturation after chemotherapy and radiotherapy in an adult patient. We also assessed that molecular SHH grouping with Illumina Human Methylation 450 k Bead Chip arrays showing that this molecular feature was stable during this maturation. We also discuss about treatment approaches in this rare situation.
Keywords
Medulloblastoma, Adult, Neuronal, Maturation, SHH.
Introduction
Medulloblastoma (MB) is the most common malignant brain tumor in children, but in adults the disease is extremely rare and accounts for less than 1% of all intracranial malignancies [1]. Recent studies have shown that MB is not a single disease but in fact comprises a collection of clinically and molecularly diverse tumor subgroups both in children and in adults. The current consensus is that there are four major subgroups named WNT, SHH, Group 3 and Group 4. These four molecular subgroups are found across all age groups, but they occur at strikingly different frequencies within each age group [2]. In adults, the SHH subgroup represents by far the largest subgroup, accounting for 57% of all tumors. Among the four histological variants classic, large cell/anaplastic (LCA), nodular/desmoplastic (ND) and MB with extensive nodularity (MBEN), the last two in most of the cases belongs to the SHH molecular subgroup. Interestingly, post-therapy neuronal maturation has been reported prevalently in ND and in MBEN and the totality of cases showing such favorable evolution has been reported in the pediatric age.
Case Study
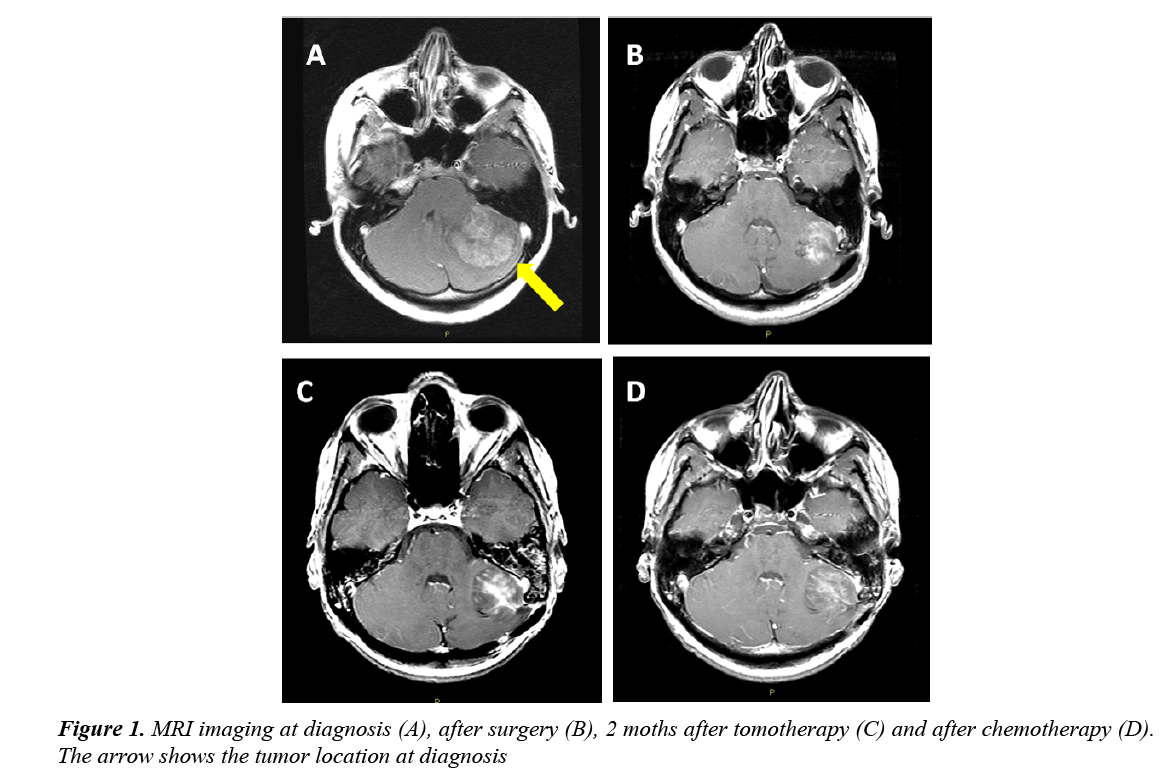
A 20 year old female patient started complaining of a headache and vomiting. A brain MRI showed the presence of a 43 × 43 mm lesion on the left hemisphere of the cerebellum, with irregular contrast enhancement and hydrocephalus (Figure 1A). The patient had a ventricular shunt put inand underwent sub-total resection of the cerebellar mass.
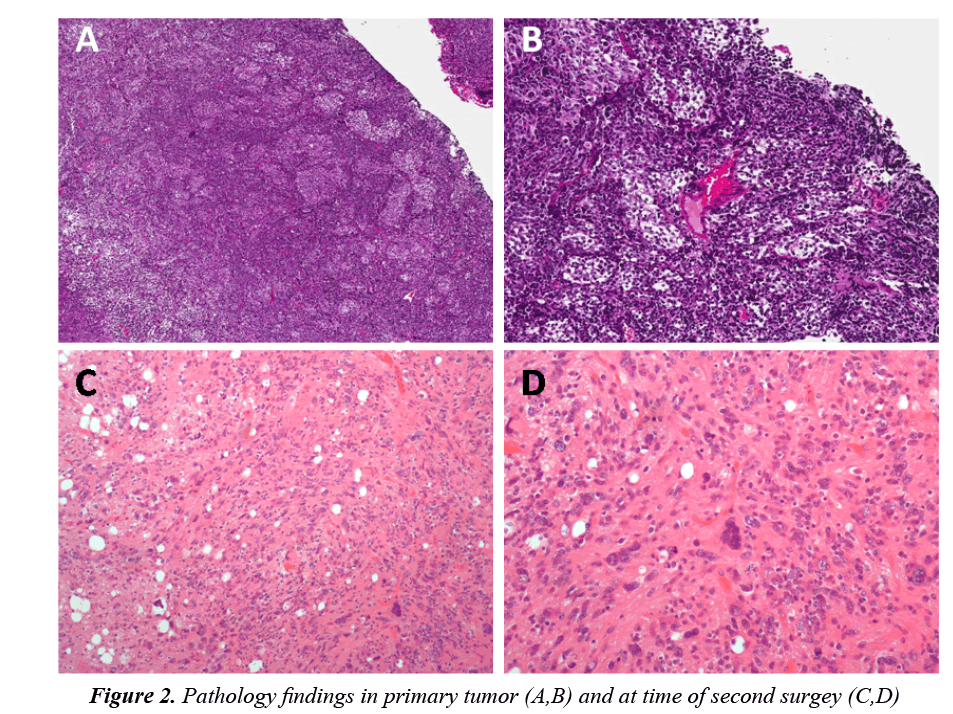
The tumor showed the histological features of a nodular/ desmoplastic medulloblastoma (MB) caracterized by pale islands surrounded by densely packed neoplastic cells (Figures 2A and 2B). The internodular areas showed frequent mitotic features with focal anaplastic changes characterized by enlarged end pleomorphic nuclei with atypical mitoses. By immunohistochemistry, the pale islands showed strong immunoreactivity for synaptophysin compared to the internodular areas. The prolifration index evaluted by Ki-67 reached 60% in the internodular areas and 3% within the nodules.
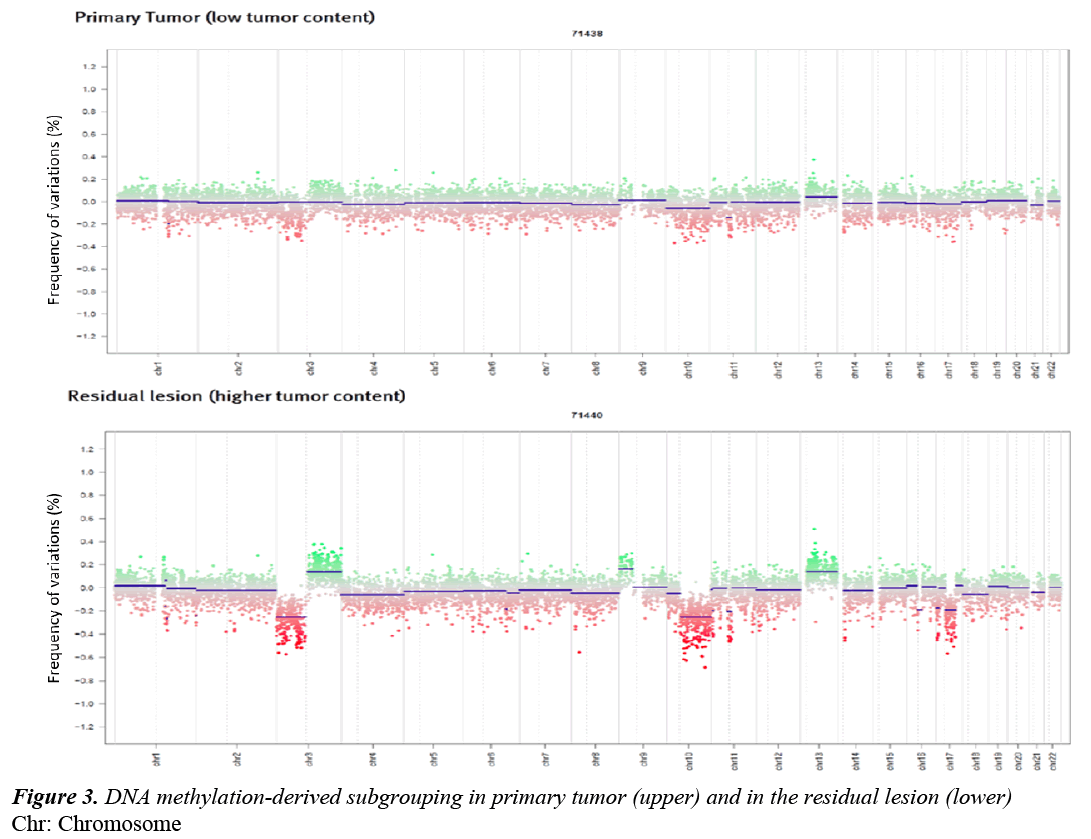
DNA methylation-derived subgroup was performed using data generated with Illumina Human Methylation 450 k BeadChip arrays as described previously [3]. Despite the low tumor cell content in the primary tumor, the lesion showed amolecular SHH profile (Figure 3).
Post-operative cranio-spinal MRI confirmed the presence of residual disease (Figure 1B) and the absence of spinal metastases. Cerebrospinal fluid was negative for neoplastic cells. The patient underwent ovaric tissue preservation and received cranio-spinal tomotherapy (32.4 Gy in 18 fractions) with a boost on posterior fossa (23.4 Gy in 17 fractions, total dose: 55.8 Gy in 32 fractions). A brain MRI performed two months after the end of tomotherapy revealed a minimal volumetric increase of the residual lesion (Figure 1C). The patient underwent two cycles of chemotherapy with Cisplatin 25 mg/m2 on days 1 to 4 and Etoposide 40 mg/m2 on days 1 to 4 every 28 days [4-8]. An MRI performed after two cycles showed no reduction inthe areas of contrast enhancement of the lesion. (Figure 1D). Given the radiological persistence of the disease, the patient underwent surgery and the removal was almost complete.
The tumor specimen obtained at the second surgery showed a neoplasm composed ofneuronal cells showing various degrees of maturation from neurocytic to ganglion cells. No embryonal cells were present. Mitoses were absent. A strong and diffuse positive staining for synaptophisin was observed, the Ki-67 proliferation index was only 2% (Figures 2C and 2D).The residual lesion showed the post-therapy neuronal maturation of a medulloblastoma. Histological examination revealed neuronal maturation of the neoplastic cells.
DNA methylation-derived subgrouping was also performed on the residual lesion and confirmed the molecular SHH profile. Moreover the specimen showed chromosomal alterations similar to the primary lesion (Figure 3).
No other treatments were delivered after the second resection. To date the patient is still alive 44months after primary surgery, with no radiological signs of disease relapse.
Discussion
Here we report a case of MB in an adult patient showing a post-therapy neuronal maturation and a favourable outcome.
Post therapeutic neuronal maturation is a rare event that can occur in some pediatric neoplasms, such as neuroblastoma and MB [9-14].
To our knowledge, this is the first case of neuronal maturation in an adult patient with MB.Moreover, none of the cases present in the literature have been investigated for the molecular subgrouping [2,15].
Neuronal maturation is associated with better prognosis in neuroblastoma, because mature neurons have a low mitotic activity and proliferation is slow or absent [9]. Most of the cases of neuronal maturation in MB reported in the literature showed a good prognosis [12,13].The mechanisms of the neuronal maturation are unknown. In most of the cases described, maturation occurred after chemotherapy, as in the case we reported. It is possible that chemotherapy determines the activation/deactivation of molecular patterns that drives immature cells toward neuronal differentiation [12].
Preclinical studies evaluated the role of retinoic acid (RA) in the induction of differentiation and blocking cell growthin MB. RA can induce neuronal differentiation in neuroblastoma cells [16]. A similar effect was also demonstrated in MB cell lines with both all-trans-retinoic acid (ATRA) and 9-cis-retinoic acid [17-19]. However, clinical data have not confirmed this background.
Treatment-induced maturation is a phenomenon that has also been observed in other tumors of embryonal origin, such as embryonal testicular germinal cells tumor (GCT). Indeed, it has been observed that in some cases, following treatment with platin, residual masses have differentiated teratomatous histology [20].
In the case we reported, the decision to avoid further treatments after the second surgery was based on different reasons. First of all, the patient had already received chemotherapy after radiotherapy. Furthermore, the diagnosis of tumor maturation, with an important reduction of proliferation index, discouraged the administration of chemotherapy, given the fact that differentiated cells are more resistant to cytotoxic agents (as has already been seen in testicular cancer maturation) [20].
Conclusion
Tumor maturation is a well known event in pediatric tumors. However, due to the rarity of medulloblastoma in adults, this phenomenon has not been described before.
However, physicians who treat adult patients with neuroectodermic pediatric tumors (i.e. PNET, medulloblastoma) should take into account that tumor maturation may occurr and that its recognition is essential to define the prognosis and to manage patients.
References
- Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci 2012; 19: 1541-1544.
- Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 2012; 123: 465-472.
- Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol 2013; 125: 913-916.
- Brandes AA, Ermani M, Amista P, et al. The treatment of adults with medulloblastoma: A prospective study. Int J Radiat Oncol Biol Phys 2003; 57: 755-761.
- Brandes AA, Franceschi E. Shedding light on adult medulloblastoma: Current management and opportunities for advances. Am Soc Clin Oncol Educ Book: e82-87.
- Brandes AA, Franceschi E, Tosoni A, et al. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 2007; 110: 2035-2041.
- Brandes AA, Franceschi E, Tosoni A, et al. Adult neuroectodermal tumors of posterior fossa (medulloblastoma) and of supratentorial sites (stPNET). Crit Rev Oncol Hematol 2009; 71: 165-179.
- Brandes AA, Paris MK. Review of the prognostic factors in medulloblastoma of children and adults. Crit Rev Oncol Hematol 2004; 50: 121-128.
- Torres LF, Grant N, Harding BN, Scaravilli F. Intracerebral neuroblastoma. Report of a case with neuronal maturation and long survival. Acta Neuropathol 1985; 68: 110-114.
- Kane W, Aronson SM. Gangliogliomatous maturation in cerebellar medulloblastoma. Acta Neuropathol 1967; 9: 273-279.
- de Chadarevian JP, Montes JL, O'Gorman AM, Freeman CR. Maturation of cerebellar neuroblastoma into ganglioneuroma with melanosis. A histologic, immunocytochemical and ultrastructural study. Cancer 1987; 59: 69-76.
- Cai DX, Mafra M, Schmidt RE, et al. Medulloblastomas with extensive post-therapy neuronal maturation: Report of two cases. J Neurosurg 2000; 93: 330-334.
- Geyer JR, Schofield D, Berger M, Milstein J. Differentiation of a primitive neuroectodermal tumor into a benign ganglioglioma. J Neurooncol 1992; 14: 237-241.
- Valvi S, Ziegler DS. Ganglioglioma arising from desmoplastic medulloblastoma: A case report and review of literature. Pediatrics 2017; 139.
- Gulino A, Arcella A, Giangaspero F. Pathological and molecular heterogeneity of medulloblastoma. Curr Opin Oncol 2008; 20: 668-675.
- Abemayor E, Sidell N. Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ Health Perspect 1989; 80: 3-15.
- Chang Q, Chen Z, You J, et al. All-trans-retinoic acid induces cell growth arrest in a human medulloblastoma cell line. J Neurooncol 2007; 84: 263-267.
- Bai R, Siu IM, Tyler BM, et al. Evaluation of retinoic acid therapy for OTX2-positive medulloblastomas. Neuro Oncol 2010; 12: 655-663.
- Gumireddy K, Sutton LN, Phillips PC, Reddy CD. All-trans-retinoic acid-induced apoptosis in human medulloblastoma: Activation of caspase-3/poly (ADP-ribose) polymerase 1 pathway. Clin Cancer Res 2003; 9: 4052-4059.
- Abada PB, Howell SB. Cisplatin induces resistance by triggering differentiation of testicular embryonal carcinoma cells. PLoS ONE 9: e87444.