Current Pediatric Research
International Journal of Pediatrics
Oral prostaglandin E1 derivative for maintaining systemic circulation in a neonate with duct-dependent congenital heart disease in a resource poor setting
Nwaneli Ifeyinwa Ezinne1, Adiele Daberechi Kenneth2*, Nwagboso Chimaobi3, Nwaneli Chibundo Uchenna4
1Department of Paediatrics, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
2Department of Paediatrics, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu State, Nigeria
3Department of Surgery, University of Calabar Teaching Hospital, Calabar, Cross-River State,Nigeria
4Department of Internal Medicine, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
- Corresponding Author:
- Adiele Daberechi Kenneth
Department of Paediatrics,
University of Nigeria Teaching Hospital,
Ituku-Ozalla,
Enugu State,
Nigeria
E-mail: daberechi.adiele@unn.edu.ng
Received: 29-May-2024, Manuscript No. AAJCP-23-102590; Editor assigned: 03-Jun-2024, AAJCP-23-102590 (PQ); Reviewed: 17-Jun-2024, QC No. AAJCP-23-102590; Revised: 21-Jun-2024, Manuscript No. AAJCP-23-102590 (R); Published: 28-Jun-2024, DOI: 10.35841/0971-9032.28.6.2282-2286
Background: In neonates with duct dependent Congenital Heart Disease (CHD), the use of Prostaglandin E1 (PGE1) primarily in parenteral form to maintain the patency of the Ductus Arteriosus (DA) is lifesaving. In settings with limited resources where parenteral PGE1 is not routinely available, neonates with duct-dependent CHD are potentially fatal if surgical intervention is not immediately feasible. After conducting a thorough literature search, only three case reports of oral PGE1 use were found.
Case report: A Nigerian male infant was diagnosed with pulmonary atresia, sinus venosus atrial septal defect and a moderately sized Patent Ductus Arteriosus (PDA) on his fourth day of life. On the ninth day of life, he displayed signs of cardiogenic shock and a repeat echocardiogram on the tenth day of life revealed that the ductus arteriosus had shrunk. Due to inaccessibility of parenteral prostaglandin E1, oral prostaglandin E1 derivative (misoprostol) was administered every 4 hours via feeding tube. The patient's cardiovascular condition steadily stabilized, and on the 21st day of life, echocardiography revealed a large PDA.
Conclusion: Oral prostaglandin E1 was effective in maintaining the patency of the ductus arteriosus and could be used as a palliative treatment in settings with limited resources.
Keywords
Patent ductus arteriosus, Newborn, Cyanotic heart disease, Cardiovascular, Prostaglandin
Abbreviations:
CHD: Congenital Heart Disease; PG: Prostaglandin; DA: Ductus Arteriosus; PDA: Patent Ductus Arteriosus; RR: Respiratory Rate; SPO2: Oxygen Saturation; LV: Left Ventricle; RV: Right Ventricle; VOT: Ventricular Outflow Tract; TAPSE: Tricuspid Annular Plane Systolic Excursion; SVC: Superior Vena Cava; IVC: Inferior Vena Cava; PA: Pulmonary Atresia; FBC: Full Blood Count; HCO3: Bicarbonate; IV: Intravenous
Introduction
The ductus arteriosus is a blood vessel connection between the large blood vessel that supplies blood to the lungs (pulmonary artery) and the large blood vessel that supplies blood to the rest of the body (aorta). In the growing fetus, it allows the majority of blood ejected from the right ventricle to bypass the nonfunctioning lungs and transfer to the aorta and then the placenta for oxygenation.
The lumen of the ductus produces endogenous prostaglandins, primarily Prostaglandin E2 (PGE2) and Prostaglandin I2 (PGI2), to maintain patency. An increase in arterial oxygen saturation and a decrease in endogenous prostaglandins facilitate the closure of the ductus at birth, which typically closes within the first day after birth. However, an open ductus is necessary for survival in certain heart conditions in which blood flow to the lungs is blocked, or in which the blood vessels supplying the lungs are switched [1-3]. In human neonates, PGE1 and PGE2 are potent dilators of the ductus arteriosus. Maintaining postnatal ductal patency in neonates with restricted pulmonary blood flow can prevent severe hypoxia, cyanosis and death [4-6]. In ductus dependent congenital heart disease, intravenous Prostaglandin E1 (PGE1) infusion is a treatment whose effectiveness has been demonstrated and thus is recommended. However, PGE1 is extremely expensive, requires continuous infusion at recommended administrative doses and is difficult to obtain at every center. When long-term use is required, these issues become more significant.
Oral PGE1, which is less expensive, can be administered on an outpatient basis, and commonly available in our environment has not been investigated routinely and a comprehensive literature search revealed only 3 reports of oral PGE1 use [7-9].
Case Presentation
A four-day-old infant presented with rapid respiration and unwillingness to breastfeed. The infant's rapid respiration was independent of position and worsened when breastfed. As his rapid breathing worsened, he refused to suckle and his mother resorted to feeding him infant formula. Observations of excessive sweating were made during feeding. The mother did not observe any bluish discoloration on the newborn's body, but did observe that he was significantly darker than his siblings at birth. Due to this, he was referred verbally by a doctor friend to our tertiary specialist clinic of the tertiary hospital in the state. The conception was unplanned and the pregnancy was unwanted, but it was accepted. The mother took several over the counter medications (unknown names) during the first two months of pregnancy due to a feeling of illness, weakness, and fever. However, after confirming her pregnancy in the third month, she discontinued all over the counter medications. In the sixth month of her pregnancy, she registered at the antenatal clinic, was compliant with her hemantinics (folic acid and fesolate), received a dose of tetanus toxoid vaccination, and did not receive intermittent preventive treatment for malaria. She did not experience any other febrile illness during her pregnancy, is not diabetic or hypertensive and is negative for retroviral diseases. The pregnancy was carried to term, and spontaneous labor lasted about seven hours [10]. He was born via vertex vaginal delivery, crying and weighing 4 kilograms. Following poor breastfeeding, he was fed infant formula with a cup and spoon every three to four hours. The formula was poorly constituted (1.5 to 2 heaped scoops to 30 ml of water, instead of 1 level scoop to 30 ml of water). Since his birth, he had not received any vaccinations. He is the first son of his parents and has two living female siblings. There was no history of heart disease in the family. Parents are small-scale merchants whose highest level of education is the senior school certificate examination.
On presentation to the specialist outpatient clinic, he was in respiratory distress (nasal flaring and subcostal recession) with a Respiratory Rate (RR) of 68 c/m, afebrile with a temperature of 36.8°C, not pale, centrally cyanosed (SPO2-87%), anicteric and no clinical signs of dehydration. He had a regular bounding pulse with a rate of 156 beats per minute and his blood pressure was 70/28 mmHg and capillary refill was instant.
An examination of the cardiovascular system revealed a hyperactive precordium with its apex at the 5th left intercostal space, lateral to the midclavicular line. A single first and second heart sound was heard. He had a grade 4 ejection systolic murmur maximal in the pulmonic area, radiating to the left infraclavicular region, as well as a pansystolic murmur maximal in the third left intercostal space [11]. The examination of the respiratory system revealed no abnormal sounds. He did not have abdominal organomegaly and his central nervous and musculoskeletal systems were normal. An antero-posterior chest radiograph showed cardiomegaly with cardiothoracic ratio of 70% and slightly prominent pulmonary vascular markings. Electrocardiogram showed normal sinus rhythm and QRS axis, with P-pulmonale (Table 1).
| Situs | Levocardia with apex directed leftward |
|---|---|
| Aortic root/valve | Normal, no regurgitation/stenosis |
| Coronary arteries | Normal |
| Left atrium | Compressed, smaller than right atrium |
| Pulmonary vein | Patent, normal flow velocities |
| Mitral valve | Bi-leaflet, good motion and coaptation, no regurgitation/stenosis |
| Left Ventricle (LV) | Compressed, smaller than right ventricle, good systolic function |
| Main pulmonaryartery | Normal sized (0.80 cm) No flow from the right Ventricular Outflow Tracts (VOT) into the MPA The blood supply to the lungs comes from a medium sized Patent Ductus Arteriosus (PDA) that connects the descending aorta to the pulmonary artery Moderate continuous left to right shunt flow noted The right and left pulmonary arteries are normal sized |
| Pulmonary valve | Thickened cusps with no opening (membranous pulmonary atresia) |
| Right atrium | Severely enlarged, prominent eustachian valve noted |
| Interatrial septum | Deviated to the left, two shunts seen with right to left flow (stretched Patent foramen ovale and 0.5 cm sinus venosus atrial septal defect) |
| Tricuspid valve | Normal structure/function, no stenosis, moderate to severe regurgitation, increased annulus dimension (1.70 cm) |
| Right Ventricle (RV) | Severely dilated and hypertrophied, good systolic function |
| Interventricular septum | Intact, deviated to the left, no ventricular septal defect |
| Systemic veins | Right superior vena cava; inferior vena cava is not distended, with good respiratory response |
| Pericardium | No effusion seen |
| LV ejection fraction | Good=70% (composite figure) |
| Cardiac output | Normal=3.24 L/min/m2 (using LVOT doppler method) |
| RV systolic function | Good. TAPSE=1.10 cm, normal for age |
| Left VOT | Normal dimension (0.70 cm), no obstruction seen |
| Right VOT | Normal RVOT 1 and 2 diameters. |
| RV systolic pressure | Severely increased=116.05 mmHg using TR jet velocity |
| Systemic veins | The right SVC and IVC drain normally into the right atrium |
| Others | Left sided aortic arch, no coarctation, no pleural effusion |
Table 1. Two dimentional echocardiogram findings are enumerated in the table below.
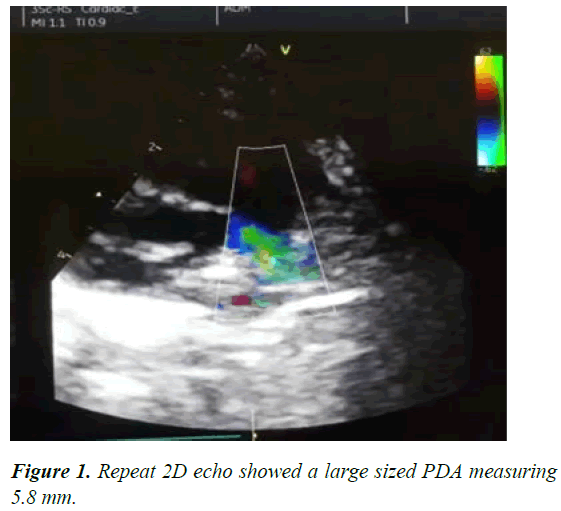
Arterial blood gas was also requested but could not be accessed. A diagnosis of Pulmonary Atresia (PA) with a moderate sized PDA was made. The baby was placed on oral frusemide at 2 mg/kg/day and enalapril at 0.05 mg/kg/day. Five days later (ninth day of life), the parents returned with complaints of cough of three days duration, worsening fast breathing of two days duration, extreme weakness and refusal to feed of a day duration. On examination, he was lethargic, in respiratory distress (RR: 22 c/m), febrile (Temperature: 37.9°C), dusky coloured, pale looking with cold extremities, anicteric, centrally and peripherally cyanosed (SPO2: 35%). Pulse was impalpable, heart rate was 168 bpm. Apex beat remained at the 6th left intercostal space lateral to midclavicular line and the cardiac mumur remained the same. He had widespread coarse crepitations, without rhonchi. He also had hepatomegaly, firm to soft and sharp eged and tenderness could not be ascertained. While being examined, he started rolling the eyeballs upwards which lasted for 5 secs; 2 episodes were obtained in 10 minutes. The pupils were equal and reactive to light and there was global hypotonia and suboptimal primitive reflexes. Diagnoses of cardiogenic shock in a neonate with PA and a likely closing PDA, and bronchopneumonia were made. Hypoglycemia and meningitis were also considered as differential diagnoses. Random blood sugar was 68 mg/dl, Full Blood Count (FBC) showed leucocytosis (38 × 109/l) with relative neutrophilia (79%), hemoglobin level was 16.1 g/dl [12]. Serum electrolytes were normal except for bicarbonate (HCO3: 18 mEq/L). The patient was placed on intranasal oxygen at 2 L/min and resuscitated using 5 mls/kg of intravenous 5% dextrose in normal saline (antishock). Other medications given were Intravenous (IV) diazepam 0.2 mg/kg stat, IV phenol barbitone loading 20 mg/kg, maintained at 2.5 mg/kg 12 hrly and IV ceftazidime at 50 mg/kg 12 hrly. Following 2 anti-shock and other resuscitative measures, radial pulse became palpable (149 bpm), SPO2: 62% to 68%, RR: 72 breaths per minute. Intravenous sodium bicarbonate 2 mmol/kg over 30 minutes and IV gentamycin 2.5 mg/kg 12 hrly were then given. Repeat 2D echocardiogram revealed reduction in the size of the PDA to approx 0.6 mm. A repeat chest X-ray showed patchy opacities consistent with broncho pnuemonia. He was subsequently placed on nasogastric tube and given 30 mls of expressed breastmilk 3 hrly when he was stable. At this time, the following drugs were added IV frusemide 2 mg/kg 12 hrly, oral enalapril 0.05 mg/kg 12 hrly, oral spironolactone 1.5 mg/ kg 12 hrly and oral digoxin 10 μg/kg/day. Intravenous misoprostol was sought was not available at the different pharmacies and hospitals. By the second day on admission, the patient’s condition had not significantly improved except for reduced episodes of cough. SPO2 varied 50% to 68% on Intranasal Oxygen (INO2), heart rate ranged between 168 to 172 beats/min and respiratory rate was 72 to 84 breaths per minute. Oral misoprostol (Cytotec®) was then compounded and given at a dose of 0.5 μg/kg 6 hourly. About 4 hours following administration of oral misoprostol, the SPO2 increased to 79% to 85% on INO2 and by the 4th day the SPO2 further increased to 88% to 91% on INO2. Respiratory rate was between 44 to 58 breaths per minute, while the pulse rate ranged between 140 to 148 beats per minute. By the 7th day on admission, the SPO2 had improved to 90% to 92% on INO2 on oxygen concentrator at 0.5 L/min and 88% to 90% on room air [13]. Repeat 2D echo showed a large sized PDA measuring 5.8 mm (Figure 1).
Five days later, the patient was transferred to a cardiac surgery center within the Stat, where a team of paediatric cardiac surgeons from the United States of America perform free cardiac surgery.
Discussion
Oral PGE1 has been found to be as effective as intravenous PGE1 in maintaining the patency of the ductus ateriosus in infants when intravenous PGE1 is unavailable or when resources are limited, but its use has not been thoroughly investigated. There are three case studies on the usage of oral PGE1 that can be found in the research literature. These case reports were from Turkey and Japan. According to these publications, far lower dosages of oral PGE1 were administered (1.5-2.0 micrograms/kg/day), in comparison to the high levels utilized for oral PGE2 and IV PGE1. The administration interval was 6 hourly which is longer than that of oral PGE2, with good results. Oral PGE1 was given to this patient at a dose of 0.5 micrograms per kilogram every 6 hours (totaling 2.0 micrograms/kg/day). This dose was identical to the dose that was given to a Japanese neonate, and the treatment was successful in both instances. In this case, the synthetic prostaglandin E1 analog misoprostol (Cytotec®) was administered orally, whereas in Japan lipo-prostaglandin E1 was utilized [14]. The oxygen saturation levels of the ten new borns who were evaluated in Turkey were significantly higher two hours after the commencement of oral PGE1 compared to the levels before the initiation of PGE; however, in our case it significantly increased after four hours. Even though resources are limited in many African countries which should be at the forefront of harnessing this innovation, there is a paucity of African literature that discusses the application of such.
Conclusion
Until intravenous PGE1 is available, resource poor facilities could begin to explore oral PGE1 as an alternative treatment option. In addition, patients who do not require hospitalization or an intravenous line could receive PGE1 orally, which is less expensive and simpler to administer. Finally, more literature on outcome of oral PGE1 use is needed.
Ethical Approval
Not applicable.
Consent for Publication
Written informed consent for publication of their clinical details was obtained from the parent of the patient. A copy of the consent form is available for review by the editor of this journal.
Availability of Data or Material
The clinical data of the patient is available upon request.
Competing Interests
The authors declare that they have no completing interests.
Funding
The case report was not funded.
Author Contributions
EIN: Conceived the idea and wrote the manuscript. AKD, NC and NCU participated in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge the VOOM foundation team for their various contributions to this work.
References
- Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev. 2018;2(2):CD011417.
[Crossref] [Google Scholar] [PubMed]
- Barst RJ, Gersony WM. The pharmacologic treatment of patent ductus arteriosus: A review of the evidence. Drugs. 1989;38(2):249-66.
[Crossref] [Google Scholar] [PubMed]
- Roehl SL, Townsend RJ. Alprostadil (Prostin VR pediatric sterile solution, the Upjohn company). Drug Intell Clin Pharm. 1982;16(11):823-32.
[Crossref] [Google Scholar] [PubMed]
- Reese J, Veldman A, Shah L, et al. Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol. 2010;34(3):222-30.
[Crossref] [Google Scholar] [PubMed]
- Olley PM, Coceani F, Bodach E. E?type prostaglandins: A new emergency therapy for certain cyanotic congenital heart malformations. Circulation. 1976;53(4):728-31.
[Crossref] [Google Scholar] [PubMed]
- Momma K, Uemura S, Nishihara S, et al. Dilatation of the ductus arteriosus by prostaglandins and prostaglandin's precursors. Pediatr Res. 1980;14(9):1074-7.
[Crossref] [Google Scholar] [PubMed]
- Annagur A, Altunhan H, Konak M, et al. 1128 Evaluation of oral prostaglandin E1 in management of ductus dependent congenital heart disease. Arch Dis Child. 2012;97(2):A323-4.
- Miyake A, Okada S, Ishikawa Y. Successful use of oral prostaglandin E1 derivative for maintaining ductus-dependent systemic circulation in a neonate with trisomy 18. Cardiol Young. 2019;29(9):1222-4.
[Crossref] [Google Scholar] [PubMed]
- Saji T, Matsuura H, Hoshino K, et al. Oral prostaglandin E1 derivative (OP-1206) in an infant with double outlet right ventricle and pulmonary stenosis. Effect on ductus-dependent pulmonary circulation. Jpn Heart J. 1991;32(5):735-40.
[Crossref] [Google Scholar] [PubMed]
- Silove ED, Coe JY, Shiu MF, et al. Oral prostaglandin E2 in ductus-dependent pulmonary circulation. Circulation. 1981;63(3):682-8.
[Crossref] [Google Scholar] [PubMed]
- Silove ED, Roberts DGV, de Giovanni JV. Evaluation of oral and low dose intravenous prostaglandin E2 in management of ductus-dependent congenital heart disease. Arch Dis Child. 1985;60(11):1025-30.
[Crossref] [Google Scholar] [PubMed]
- Lweis AB, Freed MD, Heymann MA, et al. Side effects of therapy with prostaglandin E! in infants with critical congenital heart disease. Circulation. 1981;64(5):893-8.
[Crossref] [Google Scholar] [PubMed]
- Perme T, Mali S, Vidmar I, et al. Prolonged prostaglandin E1 therapy in a neonate with pulmonary atresia and ventricular septal defect amd thedevelopmentof antralfoveolar hyperplasia and hypertrophic pyloric stenosis. Ups J Med Sci. 2013;118(2):138-42.
[Crossref] [Google Scholar] [PubMed]
- Thanopoulos BD, Andreou A, Frimas C. Prostaglandin E2 administration in infabts with ductus-dependent cyanotic congenital heart disease. Eur J Pediatr. 1987;146(3):279-82.
[Crossref] [Google Scholar] [PubMed]