Current Pediatric Research
International Journal of Pediatrics
Feasibility of minimal enteral nutrition in neonates with perinatal asphyxia during therapeutic hypothermia: A randomized controlled trial.
Trimal Kulkarni, Siddu Charki* , Vijayakumar Biradar, Tanmaya Tyagaraj, Anju T, MM Patil, SS Kalyanshettar, SV Patil
Department of Pediatrics, BLDE (DU) Shri BM Patil Medical College Hospital and Research Centre, Vijayapur, India
- Corresponding Author:
- Dr. Siddu Charki
Department of Neonatology and Pediatrics, BLDE (DU) Shri B M Patil Medical College Hospital and Research Centre, Vijayapur India
Tel: +919008002974
E-mail: drsidducharki@gmail.com
Accepted date: March 26th, 2021
Background: Therapeutic Hypothermia (TH) is a standard of care for neonates with birth asphyxia with moderate to severe hypoxic-ischemic encephalopathy. Despite lack of evidence, it is common practice to withhold feeds during TH due to risk of NEC. As there are no prior Indian studies and in view of limited evidence, this study was aimed to assess the feasibility of minimal enteral nutrition in our cohort of asphyxiated neonates during TH. Methods: This study was conducted in the Level III a NICU of Shri BM Patil Medical College Hospital and Research Centre, Vijayapura. Design: Open-label, parallel grouped, randomized controlled trial. 100 asphyxiated neonates undergoing therapeutic hypothermia were enrolled. Eligible newborns were randomized to receive either minimal enteral feeding or no feeding (50 newborns in each group) during therapeutic hypothermia. Results: Both groups were comparable in maternal and neonatal characteristics. Among 100 cooled neonates, 50 cooled neonates were randomized to MEN group and 50 cooled neonates to Control group. No statistically significant differences were observed among complications associated with therapeutic hypothermia between MEN group and Control group. Indicators of Sepsis and NEC such as Leucopenia/Neutropenia/Thrombocytopenia were observed in both groups which was not statistically significant (p>0.05). MEN neonates had a reduced length of hospital stay (mean 9 ± 2 days vs. 14 ± 3 days, p<0.05), and time to reach full oral feeds (7 ± 2 days vs. 12 ± 3 days, p<0.05). Conclusion: Minimal enteral feeding for neonates with moderate or severe HIE receiving therapeutic hypothermia is safe and feasible and associated with decreased time to reach full enteral feeding and reduced NICU stay and is not associated with significant complications like NEC.
Abstract
Background: Therapeutic Hypothermia (TH) is a standard of care for neonates with birth asphyxia with moderate to severe hypoxic-ischemic encephalopathy. Despite lack of evidence, it is common practice to withhold feeds during TH due to risk of NEC. As there are no prior Indian studies and in view of limited evidence, this study was aimed to assess the feasibility of minimal enteral nutrition in our cohort of asphyxiated neonates during TH.
Methods: This study was conducted in the Level III a NICU of Shri BM Patil Medical College Hospital and Research Centre, Vijayapura.
Design: Open-label, parallel grouped, randomized controlled trial. 100 asphyxiated neonates undergoing therapeutic hypothermia were enrolled. Eligible newborns were randomized to receive either minimal enteral feeding or no feeding (50 newborns in each group) during therapeutic hypothermia.
Results: Both groups were comparable in maternal and neonatal characteristics. Among 100 cooled neonates, 50 cooled neonates were randomized to MEN group and 50 cooled neonates to Control group. No statistically significant differences were observed among complications associated with therapeutic hypothermia between MEN group and Control group. Indicators of Sepsis and NEC such as Leucopenia/Neutropenia/Thrombocytopenia were observed in both groups which was not statistically significant (p>0.05). MEN neonates had a reduced length of hospital stay (mean 9 ± 2 days vs. 14 ± 3 days, p<0.05), and time to reach full oral feeds (7 ± 2 days vs. 12 ± 3 days, p<0.05).
Conclusion: Minimal enteral feeding for neonates with moderate or severe HIE receiving therapeutic hypothermia is safe and feasible and associated with decreased time to reach full enteral feeding and reduced NICU stay and is not associated with significant complications like NEC.
Keywords
Hypothermia, Enteral nutrition, Hypoxic-ischemic encephalopathy, Necrotizing enterocolitis, Neonate.
Introduction
Perinatal asphyxia has a burden of 10-20/1000 live births in lower and middle-income countries compared to 1-2/1000 live births in developed countries. Perinatal asphyxia causes multiorgan damage [1]. Apart from the brain, which can have long term squeal, other organs such as the kidneys, heart, gut etc. are transiently affected. Therapeutic Hypothermia (TH) is a standard of care for neonates with birth asphyxia with moderate to severe hypoxic-ischemic encephalopathy with the number needed to treat for the additional benefit being seven as per the last Cochrane review. Necrotizing Entero Colitis (NEC) is one of the concerns due to hypoxic injury to the gut. Compared to preterms, NEC in term gestation is rare [2]. In the Cochrane review amongst 11 trials, 3 trials reported data on NEC and only one child had NEC due to asphyxia despite nil per oral.2 There is little evidence to inform provision of enteral nutrition to neonates with Hypoxic Ischemic Encephalopathy (HIE) during and soon after therapeutic hypothermia; as a consequence, clinical practice is both variable and changing. Internationally, practice is even more variable: withholding enteral feeds is practiced almost universally in some countries, while in others, milk feeding during hypothermia is routine [3,4].
The epithelial lining of the gut plays a vital role in innate immunity. It prevents translocation of harmful microorganisms into the systemic circulation. Inflammatory mediators released as part of gut immunity due to stress of asphyxia can also have local and systemic effects [5]. In this context, minimal enteral nutrition can maintain both structural and functional integrity of the gut thereby reduce the systemic inflammatory response and promotes colonization and proliferation of gut micro biome. Two prior retrospective studies in this regard have shown the minimal enteral nutrition to be feasible and associated with earlier full feeding, lesser length of stay and less pro-inflammatory cytokines in the fed group [2,3,6]. However, both the studies had limited numbers and hence limited evidence exists for early introduction of minimal enteral nutrition during cooling. As there are no prior Indian studies and in view of limited evidence, this study was conducted to assess the feasibility of minimal enteral nutrition in our cohort of asphyxiated neonates during therapeutic hypothermia.
Objectives
• To assess the safety and feasibility of Minimal Enteral Nutrition in asphyxiated neonates during Therapeutic Hypothermia.
• To assess duration to reach full feeds and length of NICU stay and risk of Necrotizing Enterocolitis.
Materials and Methods
This study was conducted in the Level IIIA Neonatal Intensive Care Unit of of Shri B M Patil Medical College Hospital and Research Centre, BLDE (DU), Vijayapur.
Study design
Open labeled, parallel group, randomized controlled trial.
Study duration
1 Year (January 2020-December 2020).
Inclusion Criteria: All neonates born between January 2020 to December 2020, above 36 weeks gestation with evidence of intrapartum asphyxia (APGAR score: <5 at 10 min, Continued need for PPV at 10 min, Cord or postnatal pH before 1 hr <7.00, Cord or postnatal base deficit before 1 hr >16 mmmol/L) and presence of moderate or severe hypoxicischemic encephalopathy as per modified Sarnat and Sarnat staging underwent therapeutic hypothermia within 6 hours of life were included. Exclusion Criteria: Neonates <36 weeks and birth weight <1.8 kg with evidence of intrapartum asphyxia were excluded from the study [7,8].
Therapeutic Hypothermia Protocol: Asphyxiated neonates were enrolled and treated with Servo controlled therapeutic hypothermia as per unit Therapeutic H protocol (TOBY criteria] [9,10]. Cooling was initiated before six hours of life.
Phases of cooling
• Induction phase: to attain target core temperature of 33.50°C, it takes around 30 mins. Rectal temperature was checked every 15 min for the first hour and then hourly.
• Maintenance phase: characterized by maintenance of core [rectal] temperature between 340°C and 350°C (33.50°C) for 72 hours.
• Rewarming phase: Controlled rewarming was initiated gradually after 72 h of hypothermia at 0.50°C per hour (6 hours) from 33.50°C to 36.50°C. Continuous monitoring of vitals [blood pressure/heart rate/respiratory rate/SpO2] and glucose monitoring every 8 hours was done. Lab parameters were assessed as follows: Complete blood count-0, 48 hrs, 72 hrs, renal function tests-0, 24 hrs, 72 hrs, Coagulation profile- 0, 24 hrs, 72 hrs, Liver function tests (SGOT and SGPT)-0 and 72 hrs.
MEN protocol
The unit policy was formulated to start on minimal enteral nutrition with mother’s milk in these asphyxiated babies during therapeutic hypothermia in Protocol group, if there is no altered gastric aspirate and/or abdominal distension for babies with not on more than one inotropic support from 6 hours of life till the rewarming phase of therapeutic hypothermia. Feeds were withheld if the neonate developed feed intolerance as evidenced by one or more signs: vomiting, abdominal distension (increase in abdominal girth by 2 cms measured at umbilicus) and altered gastric aspirates.
Feeds were started at minimal volumes (10-20 mL/kg/day) and were therefore designated as MEN. The babies fed at each in situation were matched to control neonates at the same institution. Feeds were started and graded up to full feeds at the discretion of the clinical team. Collected data included demographic information, feed content and volume, length of hospital stay, time to reach full oral feeds, and feeding complications (abdominal distension, bilious residuals, etc.). Bloodstream infection was defined as a blood culture-proven infection, and necrotizing enterocolitis was defined as feeding intolerance with stage II A or higher modified Bell’s staging criteria [7].
Baseline maternal and neonatal characteristics were documented. Demographic parameters (gestational age, birth and sex), neonatal features (mode of delivery, acute intrapartum events, Apgar score at 1, 5, and 10 minutes and the need of neonatal resuscitation) were noted. Severity of HIE was assessed prior to cooling using Sarnat and Sarnat criteria. Data on feed initiation, feed increase after rewarming phase, full feed attained day and details of feed intolerance and or NEC if any will be entered in the structured proforma.
Associated complications such as Thrombocytopenia (Platelet count <1, 50,000/cumm), Coagulopathy, Hypotension, Pulmonary Arterial Hypertension (PAH), Leucopenia/ Neutropenia and Death were observed. The primary outcome measures were to analyze initiation of enteral feeds, time taken to reach full feeds, NEC and length of NICU stay. Time to reach full oral feeds was defined as the time between birth and the time when oral feedings were of sufficient quantity to result in removal of the nasogastric tube for feeding, as judged by the attending physician. Length of stay was defined as the time between admission to the unit and discharge home. The study protocol was approved by the respective ethics committees at each site.
Statistical Analysis
Standard statistical methods were used for analysis and comparison of numerical and categorical variables. Effect size and strength of association were measured using relative risk and risk difference. By univariate analysis, risk factors for the development of feed intolerance and NEC were identified. Pvalue less than 0.05 are considered to be significant. The analysis was done using statistical software packages IBMSPSS v. 23 (SPSS Inc. New York, USA).
Results
Among 279 neonates hospitalized for perinatal asphyxia in NICU during study period, 100 neonates fulfilled the criteria and received Therapeutic Hypothermia. Reasons for the 179 neonates who were not cooled include: Admission beyond 6 hours of life in 61%, mild encephalopathy in 15%, cooling contraindication in 15% and the non-availability of the cooling machine in 9%. Among 100 cooled neonates, 50 cooled neonates were randomized to MEN group and 50 cooled neonates to Control group.
Characteristics
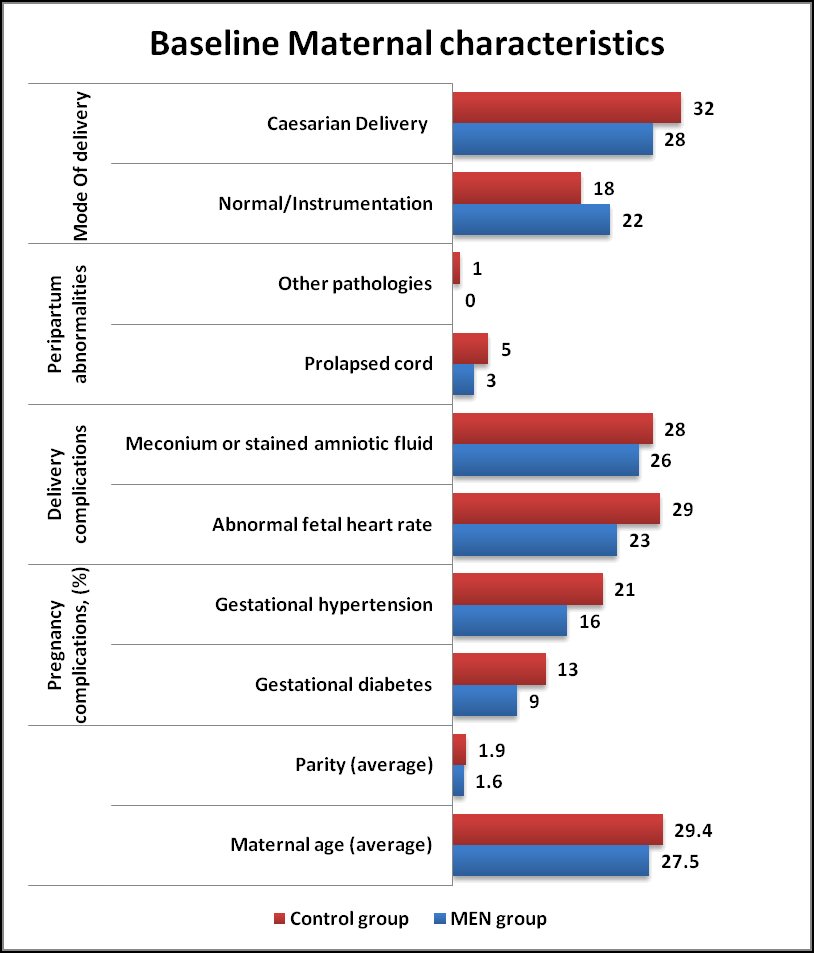
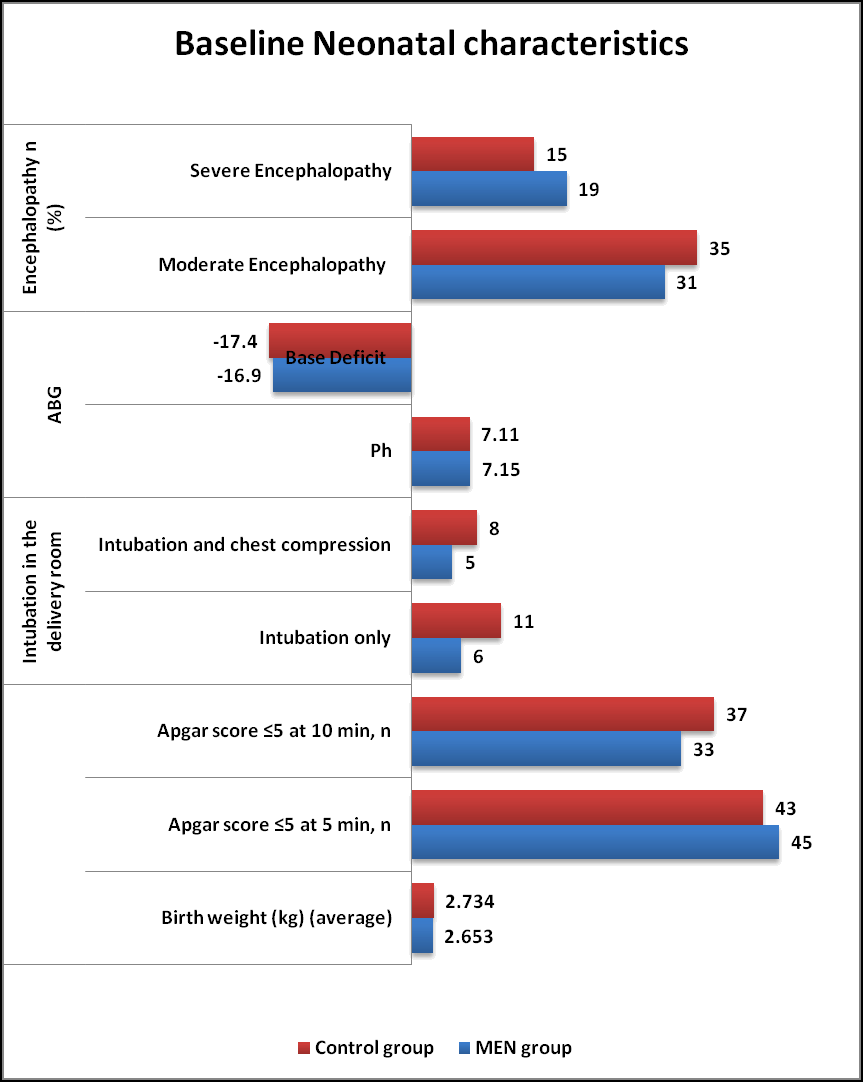
Maternal and Neonatal characteristics (Tables 1 and 2) were similar in both MEN group and Control group. The neonatal perinatal asphyxia was suspected when fetal heart rate abnormalities were noted (decelerations and bradycardia) in both groups. The HIE was classified as per Sarnat and Sarnat Staging criteria. As per criteria, Moderate Encephalopathy was seen in 62% of MEN group and 70% of Control group whereas Severe Encephalopathy in 38% of MEN group and 30% of Control group respectively in (Figures 1 and 2).
| Maternal characteristics | MEN group (n= 50) % | Controlgroup(n=50) % | P value |
|---|---|---|---|
| Maternal age (average) | 27.5 | 29.4 | NS |
| Parity (average) | 1.6 | 1.9 | NS |
| Pregnancy complications (%) | NS | ||
| Gestational diabetes | 9 | 13 | |
| Gestational hypertension | 16 | 21 | |
| Delivery complications | NS | ||
| Abnormal fetal heart rate | 23 | 29 | |
| Meconium or stained amniotic fluid | 26 | 28 | |
| Peripartum abnormalities | NS | ||
| Prolapsed cord | 3 | 5 | |
| Other pathologies | 0 | 1 | |
| Mode Of delivery | NS | ||
| Normal/Instrumentation | 22 | 18 | |
| Caesarian Delivery | 28 | 32 |
Table 1. Baseline maternal characteristics in both groups. *: Results based on chi-square (χ2) test; NS: Not Significant.
| Neonatal characteristics | MEN group (n=50) % | Control group (n=50) % | P value |
|---|---|---|---|
| Birth weight (g) (average) | 2653 | 2734 | NS |
| Apgar score ≤ 5 at 5 min, n (%) | 45 (90) | 43 (86) | NS |
| Apgar score ≤ 5 at 10 min, n (%) | 33 (66) | 37 (74) | NS |
| Intubation in the delivery room | NS | ||
| Intubation only | 06 (12) | 11 (22) | |
| Intubation and chest compression | 05 (10) | 08 (16) | |
| ABG | NS | ||
| Ph | 7.15 ± 0.78 | 7.11 ± 1.38 | |
| Base Deficit | -16.9 ± 4.4 | -17.4 ± 5.8 | |
| Encephalopathy n (%) | NS | ||
| Moderate Encephalopathy | 31 (62) | 35 (70) | |
| Severe Encephalopathy | 19 (38) | 15 (30) |
Table 2. Baseline neonatal characteristics in both groups. *: Results based on chi-square (χ2) test. NS: Not Significant.
Asphyxiated Neonates were admitted at 3.6 ± 2.6 hours of life in the NICU. Therapeutic hypothermia was initiated at 3.8 ± 1 hours of life in the MEN group and at 4.1 ± 1 hours of life in Control group. Observations of lab parameters in MEN group and Control group were documented. It shows no significant differences in Hemoglobin/Total count and Coagulation profile after 72 hours of therapeutic hypothermia in MEN group and Control group (Tables 3 and 4).
| Parameters | At admission | At 72 hours | P-value |
|---|---|---|---|
| Haemoglobin | 18.55 ± 1.048 | 16.90 ± 2.64 | Ns |
| Total leucocytes | 16996 ± 8771 | 17428 ± 5960 | Ns |
| Pt | 14.63 ± 6.23 | 16.90 ± 7.34 | Ns |
| Aptt | 49.83 ± 28.24 | 56.16 ± 31.22 | Ns |
Table 3. Observations of lab parameters in MEN Group. *: Results based on Mann-Whitney U test comparison, NS: Not Significant; S: Significant.
| Parameters | At admission | At 72 hours | P-value |
|---|---|---|---|
| Haemoglobin | 17.45 ± 2.048 | 15.90 ± 2.64 | Ns |
| Total leucocytes | 17996 ± 4771 | 15428 ± 5960 | Ns |
| Pt | 17.63 ± 9.56 | 19.52 ± 7.51 | Ns |
| Aptt | 44.81 ± 9.68 | 54.31 ± 4.57 | Ns |
Table 4. Observations of lab parameters in control group. *: Results based on Mann-Whitney U test comparison. NS: not significant S: Significant.
Complications
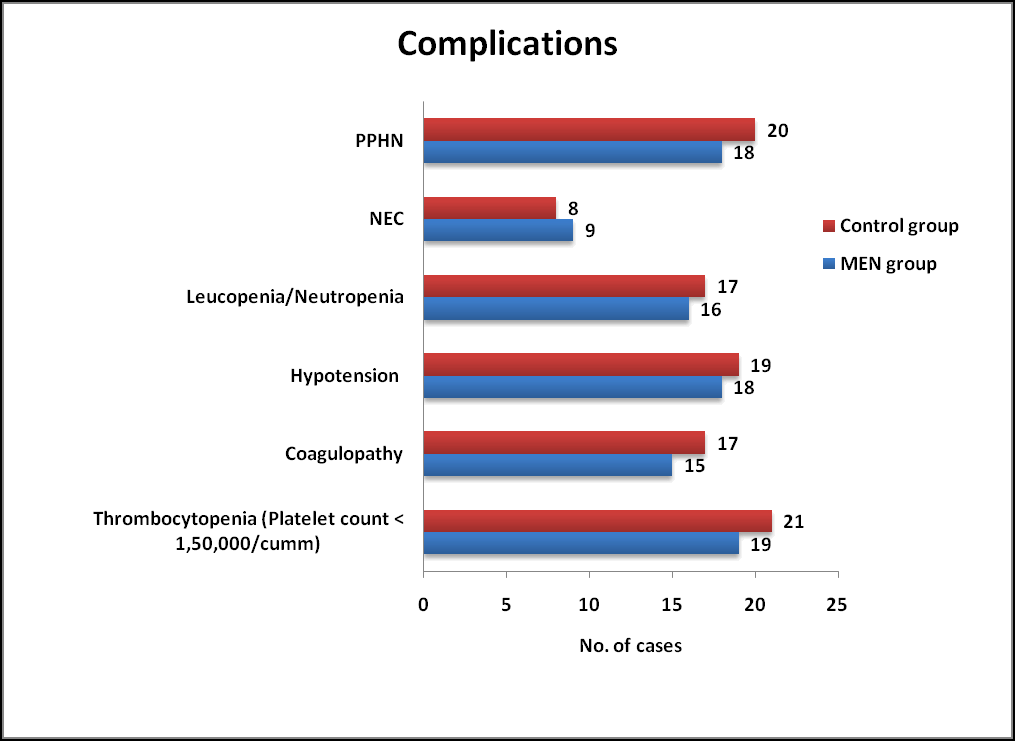
No statistically significant differences were observed among complications associated with therapeutic hypothermia between MEN group and Control group. Indicators of Sepsis and NEC such as Leucopenia/Neutropenia/Thrombocytopenia were observed in both groups which was not statistically significant. (Table 5 and Figure 3).
| Parameters | Men group (n=50) % | Control group (n=50) % | P value | P value |
|---|---|---|---|---|
| Thrombocytopenia (platelet count <1,50,000/cumm) | 19 (38) | 21 (42) | 0.683 | Ns |
| Coagulopathy | 15 (30) | 17 (34) | 0.409 | Ns |
| Hypotension | 18 (36) | 19 (38) | 0.398 | Ns |
| Leucopenia/neutropenia | 16 (32) | 17 (34) | 0.832 | Ns |
| Nec | 09 (18) | 08 (16) | 0.79 | Ns |
| Pphn | 18 (36) | 20 (40) | 0.68 | Ns |
Table 5. Complications observed among MEN and Control group. NEC: Necrotizing Enterocolitis; *: Results based on chi-square (χ2) test; NS: Not Significant; S: Significant.
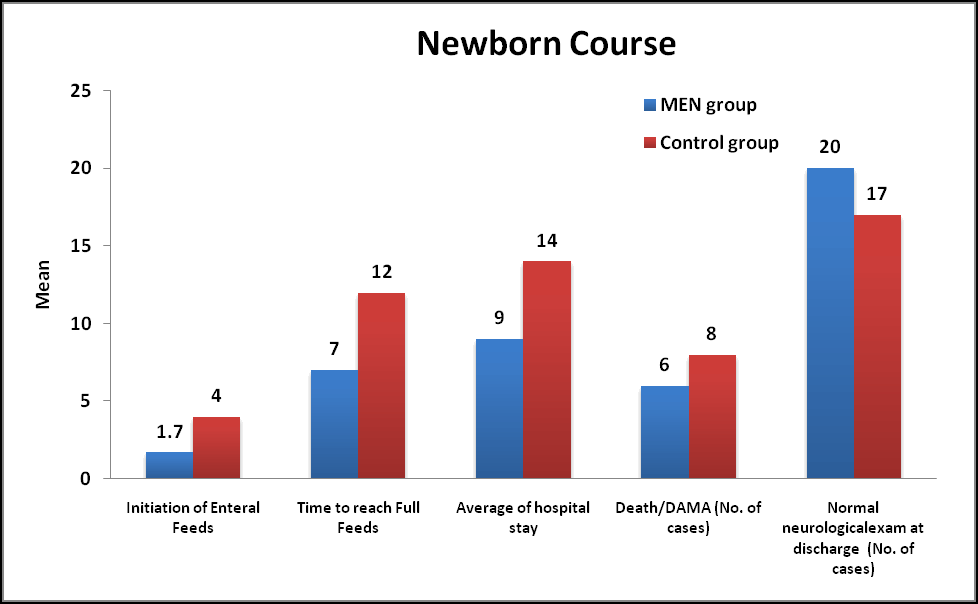
Course of Newborns: Feeding was initiated in MEN group on 1.7 ± 0.9 days of life whereas in Control group it was initiated on 4 ± 0.5 days of life which was statistically significant (Pvalue less than 0.05). Time taken to reach full feeds (100 ml/kg/day) in MEN group was 7 ± 2 days whereas it took 12 ± 3 days which was statistically significant (P value less than 0.05). Hospital stay (mean duration) was 9 ± 2 days in the MEN group whereas it was 14 ± 3 days which was statistically significant (p<0.05). 12% neonates Died/DAMA in the MEN group where as 16% in the Control group. 40% and 34% had normal neurological examination at discharge in MEN group and Control group respectively (Table 6).
| Parameters | Men group (n= 50) % | Control group (n=50) % | P value | P value |
|---|---|---|---|---|
| Initiation of enteral feeds | 1.7 ± 0.9 | 4 ± 0.5 | <0.001 | S |
| Time to reach full feeds | 7 ± 2 | 12 ± 3 | <0.001 | S |
| Average of hospital stay | 9 ± 2 | 14 ± 3 | <0.001 | S |
| Death/dama | 6 (12) | 8 (16) | 0.564 | Ns |
| Normal neurological exam at discharge | 20 (40) | 17 (34) | 0.534 | Ns |
Table 6. Newborn course during hospitalization in both groups. *: Values are represented as mean ± SD. Results based on Mann-Whitney U test comparison and Chi-square (χ2) test, NS: not significant S: Significant.
Discussion
This study demonstrated that minimal enteral feeding during therapeutic hypothermia was associated with significant reductions in the duration of the time to reach full oral feeds, and duration of hospitalization with no risk of Necrotizing Enterocolitis compared to non-feeding group. To our knowledge, this is the first RCT to study the impact of minimal enteral feeding during therapeutic hypothermia in asphyxiated neonates. Thyagarajan et al. study demonstrated that enteral feeding during therapeutic hypothermia was not associated with increased mortality or morbidity [4]. Our findings suggest that enteral feeds can be tolerated by newborns with HIE receiving therapeutic hypothermia without a significant increase in complications.
The gut plays a crucial role in the pathophysiology of critical illnesses such as HIE and NEC. The epithelial lining of the gut acts as a barrier, and plays an important role in preventing translocation of bacteria into the systemic circulation; inflammatory mediators released by the enteric immune system have local intestinal and systemic effects. In this context, enteral nutrition, particularly with mother’s own milk, may actually play a beneficial role by influencing structural and functional integrity of the gut, reducing systemic inflammatory responses and promoting proliferation of gut microbial diversity.8
Feeding tolerance is improved by gradual increase in intestinal blood flow in the first few days of postnatal life [9-11]. Mild to moderate hypothermia is known to be protective against intestinal ischemia reperfusion injury [12]. There is strong experimental evidence that therapeutic hypothermia reduces liver energy failure, attenuates lung neutrophil infiltration, preserves cardiac metabolism and prolongs survival when moderate therapeutic hypothermia is applied after intestinal ischemia reperfusion [13-15]. Therapeutic hypothermia is, in fact, now being explored as a treatment option following NEC [16]. It is imperative to carefully scrutinize all adverse events related to feeding. The HIE related mortality rate between the two groups was not significantly different and compared favorably with published literature [17].
We found no differences in major clinical conditions, such as sepsis and PPHN, which could have potentially and adversely affected enteral feeding rates. Earlier feeding may promote adequate lactation and potentially improve the chance of maintaining breastfeeding long term.
Conclusion
The literature to guide feeding practices for asphyxiated neonates undergoing therapeutic hypothermia is based on sparse data, with inherent risk of bias. This study suggests that minimal enteral feeding for neonates with moderate or severe HIE receiving therapeutic hypothermia is safe and feasible and associated with earlier time to reach full enteral feeding and earlier discharge and is not associated with significant complications. The advocacy of MEN using a standardized feeding protocol should be based on the unit feeding policy aimed at improving outcomes for neonates with HIE that receive therapeutic hypothermia.
References
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329-338.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013(1):CD003311.
- Chang LL, Wynn JL, Pacella MJ, et al. Enteral Feeding as an Adjunct to Hypothermia in Neonates with Hypoxic-Ischemic Encephalopathy. Neonatology. 2018;113(4):347-352.
- Thyagarajan B, Tillqvist E, Baral V, et al. Minimal enteral nutrition during neonatal hypothermia treatment for perinatal hypoxic-ischaemic encephalopathy is safe and feasible. Acta Paediatr. 2015;104(2):146-151.
- Stefanutti G, Pierro A, Vinardi S, et al. Moderate hypothermia protects against systemic oxidative stress in a rat model of intestinal ischemia and reperfusion injury. Shock. 2005;24(2):159-164.
- Hazeldine B, Thyagarajan B, Grant M, et al. Survey of nutritional practices during therapeutic hypothermia for hypoxic-ischaemic encephalopathy. BMJ Paediatr Open 2017; 1:e000022.
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic deci- sions based upon clinical staging. Ann Surg 1978;187:1–7.
- Ojha S, Dorling J, Battersby C, et al. Optimizing nutrition during therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed 2019; 104:F230–F231.
- Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol. 2002; 29:23-39.
- Coombs RC, Morgan ME, Durbin GM, et al. Doppler assessment of human neonatal gut blood flow velocities: postnatal adaptation and response to feeds. J Pediatr Gastroenterol Nutr. 1992; 15:6-12.
- Fang S, Kempley ST, Gamsu HR. Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed. 2001; 85:F42-5.
- Vejchapipat P, Proctor E, Ramsay A, et al. Intestinal energy metabolism after ischemia-reperfusion: Effects of moderate hypothermia and perfluorocarbons. J Pediatr Surg. 2002; 37:786-90.
- Vejchapipat P, Williams SR, Proctor E, et al. Moderate hypothermia ameliorates liver energy failure after intestinal ischaemia-reperfusion in anaesthetised rats. J Pediatr Surg. 2001; 36:269-75.
- Vinardi S, Pierro A, Parkinson EJ, et al. Hypothermia throughout intestinal ischaemia-reperfusion injury attenuates lung neutrophil infiltration. J Pediatr Surg. 2003;38:88-91;88-91.
- Stefanutti G, Vejchapipat P, Williams SR, et al. Heart energy metabolism after intestinal ischaemia and reperfusion. J Pediatr Surg. 2004; 39:179-83.
- Hall NJ, Eaton S, Peters MJ, et al. Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010; 125:e300-8.
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005; 353:1574-84.