Current Pediatric Research
International Journal of Pediatrics
Comparison of rate of sleep disorders and patterns in children with neurodevelopmental disorders with neurologically normal children
Aheed Khan*, LN Taneja, Jyoti Bhatia
Department of Pediatric, University of Delhi, New Delhi, India
- Corresponding Author:
- Aheed Khan
Department of Pediatric
University of Delhi
New Delhi
India
E-mail: aheedk@gmail.com
Received: 23-Jun-2022, Manuscript No. AAJCP-22-67525; Editor assigned: 27-Jun-2022, AAJCP-22-67525 (PQ); Reviewed: 11-Jul-2022, QC No. AAJCP-22-67525; Revised: 23-Aug-2022, Manuscript No. AAJCP-22-67525 (R); Published: 08-Sep-2022, DOI: 10.35841/0971-9032.26.8.1572-1592.
Introduction: Changing sleep patterns in children attributing to changing lifestyles and increased screen time had led to more pronounced cognitive, emotional, social and behavioural problems in children. In the Indian setup, parents are seen to be less aware of the impact of sleep during the early growing years as well as during school going age. Children suffering from psychiatric or neurodevelopmental problems are also exposed to increased sleep related problems and can augment symptoms and caregiver burden. There is paucity of research rigor in sleep problems in various neurodevelopmental disorders, especially in the Indian subtext.
Objectives: We aimed to find if there are significant sleep problems in children with neurodevelopmental disorders as compared to neurologically normal children of consecutive age in a tertiary care hospital in Delhi, India. We also intend to compare and contrast sleep disorder trends in these children.
Methods: 98 children with newly diagnosed neurodevelopmental disorder or old follow-ups were taken from the OPD/IPD of a tertiary care hospital in Delhi with consecutive 98 ages matched controls. Parents of these children were interviewed using the Sleep Disturbance Scale in Children (SDSC) and sleep scores were calculated.
Results: Student T-test and Chi-square was used for data analysis and it was reported that 73 (74.5%) children out of the 98 children recruited had profound sleep problems according to the SDSC scale and only 12 (12.2%) children out of the 98 recruited from the control group had reported sleep problems. This difference was found statistically significant (p<0.001). In our test sample, 29 children had autism spectrum disorder, 17 had Attention Deficit Hyperactivity Disorder (ADHD), 22 had cerebral palsy, 16 had global developmental delay and 14 had intellectual disability. Out of these ADHD had the maximum positive sleep score (88.2%) followed by cerebral palsy (86.3%) while the lowest was for global developmental delay (56.25%). We also found that 69 children out these 73, with a positive sleep score, were affected with disorder of initiating and maintaining sleep; which was followed by sleep wake transition disorder while the lowest number is for Sleep hyperhidrosis.
Conclusion: It is concluded that children suffering with any neurodevelopmental disorder had significantly more sleep problems than neurologically normal children. Amongst the various sleep problems in children and adolescence, we also reported that disorder of initiation and maintaining sleep leading to insomnia in these children is the most commonly observed sleep problem followed by sleep wake transition disorder. The least common sleep disorder was found to be sleep hyperhidrosis. Autism spectrum disorder, cerebral palsy and Attention Deficit Hyperactivity Disorder (ADHD) were found to be having maximum attendance, while children affected with ADHD and cerebral palsy were most gravely affected by sleep related problems.
Keywords
Intellectual disability, Deficit hyperactivity disorder, Transition disorder
Introduction
Sleep in children is an important and ardent indicator of wellbeing. It has been observed that changing sleep patterns in school going children from 6 to 18 years of age attributing to changing lifestyles and increased screen time had led to more pronounced cognitive [1], emotional and behavioural problems in children [2-4]. In the Indian setup, parents are seen to be less aware of the impact of sleep during the early growing years as well as during school going age. New parents are seen to stay awake till longer hours while significantly lacking a strict sleep time for their children. In the urban setting, children usually sleep alongside or with their parents till 5 to 6 years of age, which render them exposed to altered sleep habits, sometimes starting even in infancy. However an altering sleep habit is hardly noticed as a factor which can potentially affect behaviour and cognition in a developmentally sound child [5], unless the problem is chronic, as is seen in diseases like restless leg syndrome and parasomnias like sleep talking, sleep walking and enuresis. The attendance to paediatric psychology, sleep or neurology clinic with respect to altering sleeping habits or sleep disorders is not accurately known. This prevents early treatment options or change in caregiver or parental habits to rectify the sleep problem in their child [6].
However the impact of sleep related disorders on a child with psychiatric or neurodevelopmental problems is studied to have more pronounced effects in the overall caregiver liability and hence visit to the clinic [7]. Studies have also indicated that children suffering from psychiatric or neurodevelopmental problems are also exposed to increase sleep related problems and an increased rate to referral to paediatric neurology clinic. In the last decade an increased awareness amongst parents and caregivers has led to early identification of said sleep problems as is being accepted as an added morbidity in children with Neurodevelopmental Disorders (NDD) [8]. Studies show that prevalence of sleep problems vary from 34% to 86% in NDD children but there is significant lack of data for Indian lifestyles and how Indian dwellings have contributed to sleep problems in these children [9].
The research rigor in this regard also suffers from:
• Limited case series and cohorts.
• Lack of follow up in the Indian setup.
• Lack of awareness amongst parents for referral to developmental clinic.
This impacts the ability to draw conclusions about sleep problems in specific NDD and how a similar sleep problem in a child who is neurologically and developmentally normal varies as compared to a child with NDD. Such a lacunae affects treatment options and caters to increased morbidity in these children.
This study focussed on using a questionnaire based approach to dissect the sleep related disorders using a standard and authentic tool which sheds light on differences and similarities of sleep disorders in the NDD and Neurologically normal group. This will further identify research gaps in the field of sleep disorders in these children.
Aim and objectives
Aim: To compare the rate of sleep disorders and patterns in children with neurodevelopmental disorders with neurologically normal children.
Objectives
• Primary objective: To compare the rate of sleep disorders and patterns in children with NDD with neurologically normal children.
• Secondary objective: To study the various type of sleep disorders in different types of NDD.
Review of Literature
Neurodevelopmental Disorders (NDD)
Public health and research funding in India, especially in the under-five age group has largely focussed on communicable diseases and less attention has been given to morbidity and disability arising due to neurodevelopmental disorders. These disorders, by definition, arise from impairments or insults to the developing brain or the central nervous system in the early or late developmental period. This includes antenatal, natal, perinatal, infancy and early childhood [10].
Etiologically these insults can be ischemic, genetic, infectious, traumatic or even socio- economic; but the outcome usually results in developmental delays. The range of developmental deficits varies from very specific limitations of learning or control of executive functions to global impairments of social skills or intelligence [11]. These delays range from mild to severe in nature on the development curve of the child and can contribute to under five mortality and morbidity, increased parental concern and anxiety [12], social humiliation and lack of confidence in the child and the parents.
Current burden of NDD
There are currently no biomarkers to identify these disorders or to distinguish one from the other. They are solely differentiated on the basis of clinical presentation and they frequently cooccur [11]. In a meta-analysis done in 2018 by Bitta showed that in lower and middle income class countries, the estimate was not precise and it is greater than estimated. The prevalence for all NDD was estimated to be around 7.5 per 1000 [13], being higher for neurological disorders than for developmental disorder studies. Such a difference can be because of an increased research rigor in the field of epilepsy and other symptomatic neurological disorders which form the basis of referral of NDD children to pediatric neurology or developmental clinic. The estimates for developmental disorders observed in the earlier studies were unexpectedly low, perhaps because detection of developmental disorders such as ADHD and ASD is poor in the third world countries due to lack of tools and expertise for measuring neurodevelopment in a low resource settings [13].
Fortunately in the last decade, an increased awareness about NDD and its burden has led to significant improvement in the identification and diagnosis of different clinical presentations and had given clinicians a clearer picture on different types of NDD and their etiologies. It has also led to clinicians being able to do relevant counselling for parents and encourage them to take rehabilitation measures as soon as possible for early intervention which is the first line of defence against NDD.
Types of NDD
According to DSM-5, neurodevelopmental disorders can be divided into the following types: [11]
• Intellectual disability
• Global developmental delay and cerebral palsy
• Autism spectrum disorder
• Attention deficit hyperactivity disorder
• Communication disorder
• Specific learning disorder
• Motor disorder
• Other specified and unspecified disorders
Common sleep problems in children
Many children experience some type of sleep problem. In the Indian context, most of these are not reported to the clinician and also do not have long lasting sequelae. But there is evidence to believe that some long lasting sleep problems leads to disruption of functioning, cognition and well-being in typically developing children and hence should be the focus for research [14].
Sleep problems in children can be divided into two major categories:
• Dyssomnias disorders that result in difficulty either initiating or maintaining sleep or involve excessive sleepiness.
• Parasomnias disorders that disrupt sleep after it has been initiated but do not result in complaints of insomnia or excessive sleepiness.
Studies have shown increased frequency of pediatric referral for sleep problems which include night waking, night terrors, bruxism, sleep talking and enuresis [15]. Available data demonstrates that night waking and enuresis are significantly more frequent between 2 and 5 years of age, whereas nightmares are more frequent between 6 and 10 years of age. No differences according to age exist for the other sleep problems. It can be concluded that if an important physiological function like sleep, is greatly disturbed early in life, effects can be profound and recovery can be difficult.
Sleep in neurodevelopmental disorders
A significant increase in sleep disorders is also seen in children who were known to have psychiatric or neurodevelopmental background [16]. These disorders present with different severity in these children [15] and can range from difficulties in initiating or maintaining sleep to abnormal circadian rhythms, state specific impairments, obstructive apnoea’s, sleep related seizures or movement disorders and excessive daytime drowsiness. Parental concern and anxiety is also more in the families of these children which often leads to early referral to paediatric clinic and sometimes as the presenting symptom for these disorders along with developmental delay, for example some even form a part of the criteria for the clinical diagnosis of some disorders such as Smith-Magenis, Rett, Angel man and Prader-Willi syndrome [16].
Wiggs worked on different areas of sleep disorders in NDD children and concluded that there are not enough studies which are being conducted to raise awareness on the issue of sleep in these children, let alone sleep disorders in neurologically normal children.
We would like to describe that usage of the two terms ‘neurologically normal’ and ‘typically developing’ in this review, encompasses children who have no neurological deficits on examination and had achieved milestones at an appropriate age, in that they can be said are ‘typically developing.’ They also found out that the amount of reported problems are most likely to be the tip of the iceberg and not much is known as to how sleep problems could augment or regress therapy designed to help these children. They have evidence to believe that the quality of treatment for these children is also sub-optimal and very few studies have described and analysed the sleep disorder that underlies the symptoms of a particular neurodevelopmental disorder. Doctors and psychologists lack the proper training in sleep disorders and parents’ hesitation to seek advice for such problems adds to the current lacunae of knowledge [17].
Hence the spectrum of these diseases is less meticulously studied and adds to the problem of classifying and analysing sleep problems in NDD children. The reported sleep abnormalities can be roughly divided into the following:
• Physiological sleep irregularities e.g. REM sleep abnormalities
• Sleep disorders pertaining to certain condition, e.g. Sleep apnoea in Down’s syndrome.
• Sleep problems of unspecified origin, e.g. Irregular sleep patterns and daytime sleepiness.
• Sleep disorder contributing to the primary condition, e.g. Periodic limb movement disorder augmenting impulsivity and overactive behaviour in children with ADHD.
In our report we will focus on the common NDD reporting to the pediatric, pediatric neurology or developmental clinic in a tertiary care hospital, presenting with or without the complaints of sleep problems as the primary concern.
Sleep in children with autism spectrum disorder
Autism Spectrum Disorder (ASD) is characterised with early onset difficulties in social and communication skills and unusually restrictive repetitive behaviour and interests. Crucial for the diagnosis of ASD is [11]:
• Deficits in Social-emotional reciprocity.
• Deficits in non-verbal communication used for social interaction.
• Deficits in developing, maintaining and understanding relationships.
Severity of the disease is manifested as social communication impairment and restricted, repetitive patterns of behaviour or associated with any existing neurodevelopmental disorder or known environment or genetic cause of developmental delay [11].
ASD can present with a myriad of symptoms and comorbidities, out of which sleep disturbances have been found to have the highest prevalence rates, that is, 80% for children with ASD [18]. It has been reported that 1 in 68 individuals in the USA are diagnosed with ASD and present with the core debilitating symptoms which renders them unable to pursue daily functioning without care and support [19].
Sleep disturbances most experienced with ASD are dyssomnias which include bed resistance, delayed sleep onset, poor sleep maintenance, sleep association disorders and early morning waking’s. While other medically based sleep disturbances such as parasomnias, movement disorders, circadian rhythm sleep disorders, sleep disordered breathing occur with the same frequency as in neurologically normal children [20].
In Indian families, it is also observed that parents report sleep problems in ASD when it has increased to either disrupt cognitive functioning in the child or sleep problems itself pose difficulty in families to manage these children. There are not enough sources for correlating sleep problems and attendance to the developmental clinic in the Indian scenario, but it is seen in our setup that parents often agree having features of dyssomnias than parasomnias in a child with considerable social or communication delay, when presenting first time to a pediatric clinic.
Additionally reports in the literature suggests deranged melatonin and cortisol levels in children with ASD correlates with varying neurobiological processes which regulate sleep wake and circadian rhythm cycle. Hence melatonin as a treatment option when other behavioural measures had failed has proved to improve sleep parameters in ASD children and better day time behaviour.
Overall, it is safe to assume that children with ASD are prone to sleep problems due to their condition and isolated sleep problems occurring in these children can augment autistic symptoms. Such a scenario, if unchecked can proceed into a chronic manifestation and can even proceed to adulthood. A system for early identification of sleep problems or inadequacy is warranted and can help improve severity and impact of this disease. This can prove helpful not only for the child but also for parents and family/caregivers. This might require devising checkpoints e.g. asking parents specifically about sleeping habits of the child. Screening of developmental delay or any sleep difficulty occurring under five years of age in children brought regularly for vaccination or other visits can keep in check any progression of sleep disorder in these children.
Sleep in children with attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder or ADHD is characterised with a persistent pattern of inattention, hyperactivity and impulsivity which causes significant impairment of day to day functioning or development [11].
• Inattention: Child may lack persistence, having difficulty sustaining focus and being disorganised which is not due to defiance or lack of comprehension.
• Hyperactivity refers to excessive motor activity (such as a child running about) when it is not appropriate or excessive fidgeting, tapping or talkativeness.
• Impulsivity refers to hasty actions that occur in the moment without forethought that have high potential for harm to the individual (e.g. dart g into the street without looking). Impulsivity may reflect a desire for immediate rewards or an in ability to delay gratification. Impulsive behaviours may manifest as social intrusiveness (e.g. interrupting others excessively) and/or as making important decisions without consideration of long-term consequences (e.g. taking a job without adequate information) [11].
The prevalence of ADHD is 5.3% in children and adolescents worldwide. Parental observations of the child during awake time are often taken for diagnosis, sleep problems may be the accompanying complaint. The prevalence of sleep problems in children with ADHD is reported to be in the range of 25-55%. In a recent 2014 Australian study, 62% of children with ADHD had sleep problems and took sleep medication during the first week observation period. It is hence widely reported that sleep and ADHD can augment each other and can have a multi-faceted relationship. There is evidence to believe that children suffering from ADHD can have significant sleep problems which can aggravate hyperactivity and impaired sleep can lead to presenting symptoms of ADHD. This can even lead to misdiagnosis as the symptoms of sleep impairment can present as symptoms that are remarkably similar to those of ADHD.
The interplay of sleep and ADHD is complex as identification of a primary sleep problem leading to ADHD like symptoms needs to be ruled out before diagnosis. Furthermore, sleep is affected by psychostimulants given as therapy in these children. These medications can improve sleep in some patients while can impair in other. Henceforth there is a need to identify and isolate a primary sleep disorder manifesting as ADHD like symptoms before starting pharmacotherapy for a child. Behavioural therapy targeting sleep patterns in these children can help improve symptoms and should be taken as a part of initial management of ADHD.
Sleep disordered breathing disorders, obstructive sleep apnoea, periodic limb movement disorder and circadian rhythm disorders are some of the common manifestation of sleep problems presenting in ADHD. A recent systematic study reported that the prevalence of OSA is higher in ADHD (25-30% than in the general population, which is 3%. There is also evidence to suggest that there is a delayed pattern of melatonin secretion in children with ADHD compared with controls which can lead to misalignment of sleep patterns.
Allan Hvolby had described three scenarios in the association model of sleep with ADHD in a 2015 review. The first scenario is when ADHD results in a sleep disorder and treating the disease may alleviate sleep problems. In the second scenario, a primary sleep disorder is responsible for daytime symptoms and functional impairments that are characteristic of ADHD. Treating the sleep disorder can alleviate ADHD or ADHD like symptoms; e.g. surgical adenoidectomy in OSA patients improves sleep and hence hyperactivity. In the third scenario, an underlying medical or psychiatric disorder can contribute to both sleep patterns and ADHD. Sleep problems can then form a positive feedback loop with the disease and can lead to exacerbate symptoms of each other. An integrated and complex treatment plan is needed in such a condition, often involving pharmacotherapy and managing the sleep disorder more closely.
Sleep in children with intellectual disability
Intellectual Disability (ID or Intellectual Developmental Disorder (IDD includes both intellectual and adaptive functioning deficits in conceptual, social and practical domains [11]. The following three criteria should be met:
• Deficits in intellectual functioning.
• Deficits in adaptive functioning that result in failure to meet developmental and socio cultural standards for personal independence and social responsibility.
• Onset of intellectual and adaptive deficits during the developmental period, that is before 18 years or adulthood.
Sleep problems in IDD are rarely reported as an isolated morbidity by parents. Parents seek professional help for behavioural or other chronic manifestations of intellectual disability like hyperactivity, bladder and bowel habits, obsessional states and learning difficulties and elect to keep sleep problems at home. Hence sleep or nocturnal problems are not often presented to the clinic. This pays an important aspect in our study since parents who reported with these problems, upon history taking revealed significant sleep pattern alterations in the child or in the parent/caregiver. Literature suggests that children with IDD are particularly vulnerable to sleep problems as we have discussed earlier in this review. A 2012 study revealed prevalence of sleep problems in adults with ID ranged from 8.5% to 34.1%. A prevalence of 9.2% was reported for significant sleep problems. In children with ID, prevalence rates ranged between 25.5% and 36.2% for sleep problems with an average duration between 6 and 9 years. Hence children with IDD succumb to more sleep pattern abnormalities than adults and require a more vigilant approach.
It is also reported that children who have more significant sleep problems have more profound levels of ID and require more medication to counteract symptoms. Increased possibilities of epilepsy, a greater frequency of cerebral palsy, more daytime drowsiness and daytime napping is also seen in children with IDD with severe sleep problems, as compared to IDD children who did not have a severe sleep problem. Furthermore, children with a severe sleep problem showed more severe levels of daytime problem behaviours; for example, aggression, non-compliance and hyperactivity.
Disturbances in initiating and maintaining sleep, night awaking, excessive daytime sleepiness, sleep disordered breathing and settling problems are more commonly seen than other parasomnias in IDD. In downs syndrome, which is the most common genetic cause of ID, obstructive sleep apnoea is more commonly seen due to craniofacial abnormality. It is also reported that there is an increased prevalence of upper airway obstruction and hence obstructive sleep apnoea in these children.
There is evidence to believe that lifestyle modification strategies should be considered while managing IDD as sleep behaviour can prove to be an early indicator for predicting comorbidities like overweight/obesity and sedentary behaviour in adolescents with intellectual deficiency.
Molecular basis of sleep problems have been linked to a myriad of genes, but an interesting Oxford study done in 2017 linked ID and developmental delay to a glutamate receptor encoded by GRIA1 gene. Glutamate pays a key role in the regulation of sleep. It is also the principal neurotransmitter of the retinohypothalamic tract which conveys light information from eye to the suprachiasmatic nucleus clock. They found that by inducing similar mutation in mice, as found in the IDD patients in the GRIA3 gene, leads to prolong wakefulness in mice and disrupts the organisation of sleep and wake bouts. This can implicate treatment strategies in children with severe ID who present with prolonged wakefulness and sleep problems.
Sleep in children with cerebral palsy
Cerebral palsy is a motor disorder resulting from a nonprogressive insult to the developing brain. It presents clinically with multiple debilitating conditions which had emerged due to cerebral cortical or sub-cortical insults, more commonly during the first year of life. The worldwide incidence of CP is approximately 2 to 2.5/1000 live births. Children with CP usually present with developmental delay and motor deficits. Motor deficits of CP include negative phenomena such as weakness, fatigue, incoordination and positive phenomena such as spasticity, clonus, rigidity and spasms. Spasticity is a velocity dependent increased muscle tone with hyperreflexia and can lead to muscle stiffness, functional impairment and atrophy. If not treated, it can progress to muscle fibrosis, contractures and subsequent musculoskeletal deformities. CP can be classified in many ways.
• Based on motor deficits: Mild, moderate and severe.
• Based on physiology: Cortical (pyramidal), resulting in spasticity. Spastic CP is the most common type, accounting for up to 75% of cases.
• Basal ganglial (extrapyramidal), resulting in abnormal movements such as choreoathetosis,
• Cerebellar, resulting in hypotonia, mixed.
• Etiologically, it can be classified in many ways. It can be divided according to the timing of the insult as prenatal (commonest), natal or postnatal. Another etiologic classification system depends on the actual cause such as congenital (developmental, malformations, syndromic) or acquired (traumatic, infectious, hypoxic, ischemic, torch infections and others).
• It can be classified according to the topographic distribution of motor deficit as monoplegia, diplegia, hemiplegia, triplegia, quadriplegia and double hemiplegia. However it is difficult to delineate these clinically in some children.
Children with Cerebral palsy also present with a lot of other co-morbidities like visual and hearing loss, gastrointestinal issues, undernourishment, growth restriction, drooling, epilepsy, bladder and bowel dysfunction and sleep problems. We are restricting our discussion to sleep deficiency and other sleep related disorders seen in these children. Parents and caregivers had to consult a wide variety of experts for the welfare of the CP child and this poses an additional burden on family and support provided to the child. It is often seen in the Indian setup that the propensity and chances of neglect can increase if there are increased co-morbidities in the CP child.
Lack of awareness can be attributed to neglect but parents who strive to provide adequate care for their affected child may have sleep disorders themselves. Hence, it is important for professionals who are in most frequent contact with these patients, such as rehabilitation therapists and educational psychologists, to have some basic knowledge of sleep issues related to cerebral palsy so they can recognise red flags and refer to specialists within the paediatric treatment team.
Sleep disturbance is reported to be very common in children with cerebral palsy and between 23% to 46% of children with cerebral palsy suffer from sleep problems. These include: difficulty in initiating and maintaining sleep, sleep wake transition, sleep breathing disorders and sleep bruxism, excessive day time sleeping, nightmares and sleep talking. Visual impairments leading to abnormal light perception can also aid in sleep related disorders in these children. There may be other factors such as pain, aspiration, glossoptosis, epilepsy or spasticity which might lead to altered sleep behaviour in these children.
An important take home message in clinicians who come across children with cerebral palsy might be an endeavour to screen them for not only co-morbidities previously mentioned with it but also to screen for sleep related problems. A simple sleep screening tool which can be used for evaluation of sleep in children is BEARS (B is bed time problems, E is excessive day time sleepiness, A is awakenings during the night, R is regularity as well as duration of sleep, and S is snoring) as discussed by Bharti. This could provide a holistic approach for the clinicians to manage these children at the very first visit.
Sleep in children with GDD
Global Developmental Delay (GDD) is defined as a delay in two or more developmental domains of gross/fine motor, speech/language, cognition, social/personal and activities of daily living, affecting children under the age of 5 years. Global developmental delay and intellectual disability are phenotypically and genetically heterogeneous and the term GDD is restricted primarily for early childhood because of lack of reliable assessment or for children who are too young to participate in standardised testing. A detailed evaluation and plan for investigating the child is an important step in the line of management.
The degree of developmental delay is further sub-classified as: mild (functional age <33% below chronological age), moderate (functional age 34%-66% of chronological age) and severe (functional age less than 66% of chronological age). GDD is considered significant when there is a deficit in performance of at least 2 SD below the age appropriate mean on accepted standardised assessment tests. With a prevalence of 1%-3% in children 5 years or younger, GDD is one of the most common conditions encountered in paediatrics with genetic and structural brain abnormalities being the most frequent causes. However GDD serve as an umbrella term which is used for a large variety of conditions which may or may not be diagnosed much later in life.
Irrespective of the aetiology of the delay, concerns about developmental delay is often been seen in parents of children as young as three months old. Hence a careful history and examination is the key to provide a direction for managing and accessing GDD. Hence, asking parents about sleep habits of the child can provide useful clues to rule out any sleep related problems precipitating or aggravating the current symptoms and help mitigate in caregiver burden and their own personal sleep habits. There is evidence to believe that young children with developmental delays experience sleep disorders at a higher rate than do typically developing children. The most common types of sleep disorders are found to be difficulties in initiating or maintaining sleep and sleep disordered breathing. However sleep problems in rare genetic and metabolic conditions like fragile X syndrome, or inborn errors of metabolism haven’t underwent significant research and our knowledge in that area remains thin.
There was paucity of research in the prevalence rates of sleep problems in GDD. However Bonuck and Grant in a 2012 review have discussed that difficulties in initiating or maintaining sleep are more prevalent in children with atypical development and are associated with inadequate and fragmented sleep, which can have serious behavioural and cognitive consequences and can contribute to family stress. The aetiology of difficulties with initiating or maintaining sleep in infants and toddlers are primarily behavioural, such as poor parental limit setting, inconsistent bedtime routines, or dependence upon excessive sensory stimuli. Social and environmental factors, such as crowding, noise and shared sleeping arrangements also contribute to the same. Given the importance of early intervention in these children and the likely impact of sleep disturbances on cognitive and behavioural functioning, health professionals need to become increasingly aware of and knowledgeable about sleep and sleep disorders. We all spend about one third of our lives sleeping, or trying to sleep; thus, we should understand as much as we can about it.
Sleep assessment
Our current knowledge on sleep problems in children is confined to the tell-tale experiences of the caregiver of the child and is hence less reliable. Most methods of sleep assessment rest on personal and subjective skills of the caregiver and it is difficult to obtain an objective means to assess sleep in our patients. This is increasingly common in a country like India where parents are either unaware of the condition of their child or are not educated enough to understand the gravity of sleep problems in their child. Initial assessment may include a detailed history of the child’s present ailment and reason for visit to the clinic followed by history of sleep habits and behaviours. There are both subjective and objectives measures to assess sleep habits. Subjective methods involve use of parental knowledge and personal experiences through the use of sleep questionnaires and sleep diaries. However objective methods rely less on the parents and instead directly measure aspects of sleep through various technologies. These include polysomnography, autography and video-recording. Here we will briefly describe some of the common objective and subjective methods of sleep assessment and then discuss in detail of the use of our standard questionnaire tool [19].
Sleep questionnaires: Due to social communication deficits and general developmental limitations, most of the information about sleep in children with neurodevelopmental disorders is gained from parent report. There are numerous sleep questionnaires that have been used to assess sleep in pediatric populations. Here we discuss the most common ones and briefly describe them.
The Children’s Sleep Habit Questionnaire (CSHQ): It was developed to assess sleep problems in children aged 4 to 10 years old, based on the paediatric international classification of sleep disorders. Owens and colleagues evaluated the psychometric properties of the CSHQ in a sample of school aged typically developing children with a diagnosed sleep disorder and a community sample of healthy children and found that the CSHQ demonstrated adequate internal consistency and acceptable test retest reliability for both samples [19].
Sleep Disturbance Scale for children (SDSC): Is a 26 items Likert-type rating scale (Sleep Disturbance Scale for Children: SDSC) which assesses the sleep problems in children from 2 to 16 years of age for sleep problems in the last 6 months. It covers 6 most common sleep disorders of childhood and adolescence, which are Disorder in Initiating and Maintaining Sleep (DIMS), Sleep Breathing Disorders (SBD), Disorders of Excessive Somnolence (DES), Sleep Wake Transition Disorder (SWTD), Disorders of Arousal (DA) and Sleep Hyperhydrosis (SHY).
Bruni et al. chose a 6 months period because of the low frequency in the population of some sleep disorders and in order to discriminate between transient and persistent disturbances. The questionnaire consisted of two sections: The first one was used to obtain demographic, behavioural and clinical data, information about previous illnesses and present medical status with specific questions regarding pathology that could affect sleep; the second was made up of 26 items in a likert-type scale with values 1-5 with the wording arranged so that higher numerical values reflected a greater clinical severity of symptoms. The cut-off point was taken to be at 39, which means children having a score greater or equal to 39 might be more prone to increased sleep disorders.
SDSC was made in order to: (1) Provide a standardised measure of sleep disturbance in childhood and adolescence through an easy to use sleep index score for clinicians and researchers; (2) Create a database of a large population in order to define normal values; (3) Define different subsets of items that could be used as a screening test for identifying the specific areas of sleep disorders and (4) Identify children with disturbed sleep.
The items were derived from clinical experience and from a review of previous sleep questionnaires reported in the literature. We chose SDSC for our study because of its relatively quick and easy language setting, an approachable parent-understandable format and relatable questions as compared to other sleep questionnaires models. We found evidence that the sleep disturbance scale for children may be a more appropriate measure when assessing clinical samples and personal experience also pointed an easy framework around it. It is observed that the bias concerning the information given by the parents could be minimised with the correct wording of the items and SDSC provides the same.
The Modified Simonds and Parraga Sleep Questionnaire (MSPSQ): It is a screening tool for sleep disturbances in children aged 5 to 18 years old and is composed of 51 items. It is structured in two parts: Part 1 targets sleep quantity and quality and part 2 targets specific sleep disorders. The 36 items scored on a likert scale pertain to six common sleep problem categories: bedtime resistance/struggles, sleep onset delay, parasomnias, sleep disordered breathing, sleep anxiety and daytime sleepiness. A Composite Sleep Index (CSI) is calculated based on the frequency of problems with settling at bedtime, night waking, early morning waking, co-sleeping, as well as on the duration of settling and night waking. The resulting CSI score ranges from 0 to 12 and a score of 4 or greater indicate a severe sleep problem. The measure includes additional questions to gather information on treatment history for sleep disturbance and the impact of sleep on family functioning [19].
The Family Inventory of Sleep Habits (FISH): It is another useful measure of sleep that was developed to assess sleep hygiene in children with autism from ages 3 to 10 years. This instrument gathers information about daytime habits [19].
Sleep diaries: Sleep diaries are another method commonly used to gather information from parents regarding their child’s daily sleep behaviours and pre-bedtime practices. These logs prompt parents to make nightly recordings of specific sleep related information, including: the time at which the child goes to bed, the time at which the child falls asleep, the time and frequency of night awakenings, the time at which the child wakes in the morning and the times of daytime naps [19].
In comparison to sleep questionnaires, sleep diaries provide the added benefit of collecting information at multiple time points over a period of time and do not require parents to retrospectively recall information confounded by memory and other preceding factors. Sleep diaries may be perceived as laborious by some parents and are vulnerable to reporter bias [19].
Actigraphy: An actigraph is a portable, watch like accelerometer device that detects limb movement, which the actigraph uses as a proxy to measure various parameters of the sleep wake cycle [19]. Data collected by the watch is then analyzed using computer software with specialized, age based algorithms. Actigraph can be costly, are at risk of being lost, are prone to malfunction and need to be retrieved for data downloading and data processing. Hence, actigraphy use may be less practical in most clinical settings and in large clinical research trials. At present, there is a lack of standardization across studies with regard to the brand of actigraph, analysis software and scoring algorithms. Some of the disadvantages of actigraphy include parental dependence to appropriately and consistently place the watch on the child before the child goes to bed. This might be difficult or liable to forget. Actigraphy relies on limb movement as a proxy of sleep; therefore discrepancies between actigraphy and other sleep measures may be anticipated [19].
Polysomnography: Polysomnography (PSG) is an objective measure of moment to moment sleep architecture that is typically performed in a sleep laboratory. Considered the “gold standard” in the assessment of sleep problems. PSG involves the recording of many physiological parameters including electroencephalogram, electrooculogram and electromyogram, as well as electrocardiogram, respiratory measures and leg muscle activity. PSG can identify sleep related problems that are generally undetectable or less reliably detectable by other measures, including problems specific to stages of sleep, sleep latency, total sleep duration and sleep disordered breathing.
Limitations of PSG include increased cost and since multiple electrodes need to be placed on the child, it can negatively impact sleep.
Videosomnography: Videosomnography is a portable timelapse video recording system used to gather objective data on a range of sleep behaviours, including sleep wake states, frequency and duration of night waking and other behaviours that one would expect to observe during sleeping periods (e.g. signs of obstructive sleep apnea, limb movements, parasomnias). This method can prove useful than other methods like PSG or actigraphy because it can be done in the home and evidence supports that children tolerate this method better. Limitations can be that the lights used can be distractive to the child or the child may sleep under the covers rendering it useless. Videosomnography might not detect changes as objectively as actigraph or PSG and hence is relatively used less in research. Although it can augment results from different sleep assessment methods.
Lacunae in Literature
There were a lot of evidence based articles on the importance of diagnosing and managing sleep disorders in children suffering with Neurodevelopmental disorders and they have posed emphasis on the management of sleep problems in these children. However, we found that sleep trends in Indian children has not been reported in as much detail as the west. There was significant research lacunae found in the field of assessing the sleep environment, parent education and awareness and prevalence of sleep problems reporting to the clinician in the Indian setup.
The few studies which were found assessing the said research question focussed on age specific and community specific sleep problems and were found to confine to adolescent age group than on neurodevelopmental disorders.
We also observed a significant lack of research in the application of parent centric models for sleep assessment. Screening practices for clinicians in the developmental clinic has not been described in detail. There were still a few articles describing sleep problems in NDD children in the Indian family but there were fewer or none on comparison of various sleep disorders in these children.
Our study aims to fills these lacunae by comparing the sleep disorders in the various NDD groups as well as gives a rough picture on the various types of sleep problems occurring in these children. However further research is required in that area.
Materials and Methods
Study site: Department of paediatrics, max super speciality hospital, Patparganj, Delhi, India.
Study population: Paediatric patients 2 years-16 years, diagnosed with NDD on out-patient or who may get hospitalized (in-patient).
Inclusion criteria:
• For cases: Children from age 2 years-16 years who are diagnosed to have NDD.
• For Controls: Children from 2-16 years of age who present to pediatric OPD or IPD for any other problem and are neurologically and developmentally normal.
Exclusion criteria for cases and controls: Children with any other systemic chronic illness like asthma, liver or renal disease, congenital, acquired heart disease or any chronic disease.
Sample size: The main objective of the study is to compare sleep disturbance scores in children with NDD and neurologically normal children. Among the many parameters that can be measured, sample size calculation is based on Disorders of Initiating and Maintaining Sleep (DIMS) scores in these two groups. Study done by Zuculo reported an average score of 13.19 in CP patients and 11.80 in controls using the SDSC questionnaire. They have not reported the respective standard deviation, but have reported the Standard error of mean and sample sizes. Using these values, we computed the standard deviations as 5.836 in NDD children and 3.963 in control subjects. With these values and the following formula, the sample size comes to 98 in each group.
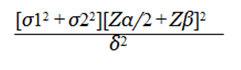
Where, Zα/2=1.96, corresponding to 95% confidence interval (Standard Error in cases) Zβ= 0.84 (standard error in controls).
σ1=5.836 (standard deviation in cases) σ2=3.963 (standard deviation in controls) δ2 (margin of error).
Participants: We recruited 98 children, from age 2 to 16 years, over a period of 18 months starting from March 2018, who were attending the outpatient department clinic of a tertiary care hospital in Delhi after taking due ethical clearance from the hospital ethics committee. These children reported to the developmental clinic or paediatric clinic majorly for complaints like developmental delay or were already diagnosed by their own paediatrician and were taken into the study while follow up. A thorough history taking and examination was done by the principal researcher as well as their own paediatrician in the clinic. Children were diagnosed with respective neurodevelopmental disorder by the paediatrician or by developmental paediatrician and was then included in the study after taking Informed consent from each primary caregiver.
Children who were diagnosed with a particular NDD constituted the ‘cases’ group. For the ‘control group,’ 98 consecutive age matched children were recruited into the study, in a similar manner, during the same duration. These children attended the general paediatric clinic or were admitted in the inpatient department for other complaints. Proper history and examination was done for the control group to exclude children who had history of systemic chronic illnesses e.g. asthma, liver or renal disease, congenital, acquired heart disease or any other chronic disease which might cause sleep problems. These children were hence typically developing and neurologically normal.
Measures: For sleep assessment we took into consideration the Sleep Disturbance Scale in Children (SDSC). It is a 26 item Likert-type rating scale which assesses the sleep problems in children in the last 6 months. It covers 6 most common sleep disorders of childhood and adolescence, which are Disorder in Initiating and Maintaining Sleep (DIMS), Sleep Breathing Disorders (SBD), Disorders of Excessive Somnolence (DES), Sleep Wake Transition Disorder (SWTD), Disorders of Arousal (DA) and Sleep Hyperhydrosis (SHY).
We chose SDSC for our study because of its relatively quick and easy language setting, an approachable parentunderstandable format and relatable questions as compared to other sleep questionnaires models, Bruni et al found that:
• SDSC is an easy to fill form to collect data on sleep behaviour of children and adolescents.
• The internal consistency is good in spite of the relative heterogeneity of the items.
• Both item and total scores do not change significantly when tested and retested.
• The six factors extracted, representing the most common areas of sleep disorders in childhood and adolescence,could be used to design a ‘child‘s sleep disturbance profile’ and would be useful for directing the clinician toward specific areas of dysfunction that require further investigation.
• The evaluation of the clinical groups using factor scores confirms the discriminating capacity of the scales derived from factor analysis.
Their analysis showed that SDSC gave a good diagnostic accuracy (AUC=0.91) and that the cut-off score with the best diagnostic confidence, as determined by the intersect point of sensitivity and specificity, was 39 (which corresponded to the upper quartile of the control group’s SDSC total score); this cut-off point gave a sensitivity of 0.89 and a specificity of 0.74.
Procedure: Ethics approval was obtained through the hospital ethics committee. In accordance, parents were provided with informed consent forms, in their best understandable language. Following the consent process, parents were asked questions consisting of demographic questions (e.g. date of birth) and medical history (e.g. details of diagnoses and health services attended). They were then asked questions regarding the sleep profile of the child using the SDSC scale.
Data analysis: For statistical analysis, student-t test and Chi square test was done, with the aid of Python and SPSS software respectively. The test for significance was repeated to confirm reliability of our data. The total score for the participants in each of the two groups was tabulated and compared using a cut-off for a positive sleep disorder been taken at 39.
Given a relatively small sample for comparing the type of sleep disorder in respective neurodevelopmental disorder, mean and standard deviation was also calculated test to identify the frequency of the six sleep disorders in each NDD.
Results
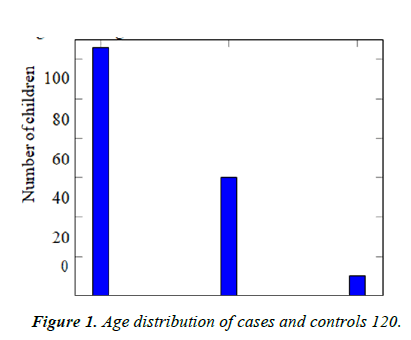
Participant characteristics: Our study recruited 98 children with NDD and 98 age matched controls. Out of these, 126 (64.3%) belonged to the age group between 2 to 5 years, 60 (30.6%) from ages 5 to 10 years and 10 (5.1%) belonged to the age group from 10 to 16 years (Table 1 and Figure 1).
| 2 to 5 years | 5 to 10 years | 10 to 16 years | Total |
|---|---|---|---|
| 126 | 60 | 10 | 196 |
Table 1. Age distribution of cases and controls.
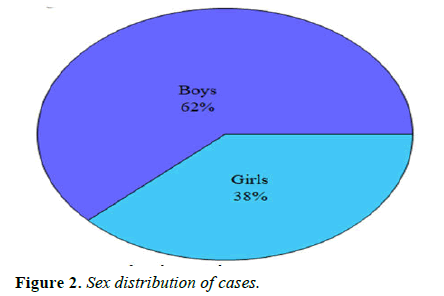
Cases: Ninety-eight children were taken as the NDD or case group, out of which 61 (62% were boys and 37 (32% were girls. These children presented to the out-patient or in-patient department with complaints of developmental delay along with other co-morbidities (Table 2 and Figure 2).
| Boys | Girls | Total |
|---|---|---|
| 61 | 37 | 98 |
Table 2. Sex distribution of cases.
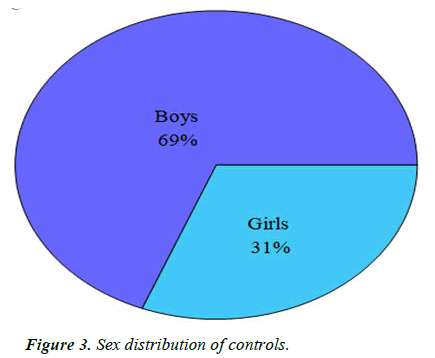
Controls: For the control or typically developing and neurologically normal children, we took consecutive age matched children who presented to the out-patient of in-patient department with complaints other than developmental delay, were developing normally and were normal neurologically. Out of these, 68 (69%) were boys and 30 (31%) were girls (Table 3 and Figure 3).
| Boys | Girls | Total |
|---|---|---|
| 68 | 30 | 98 |
Table 3. Sex distribution of controls.
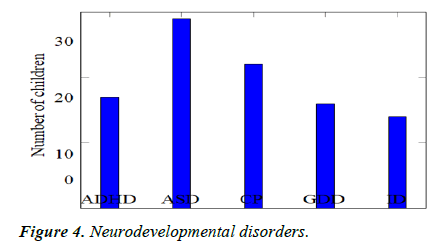
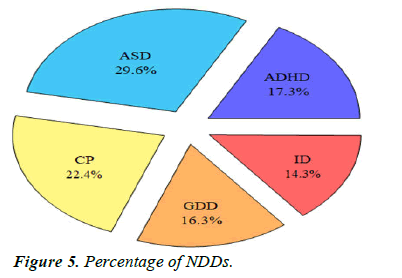
Comparison of sleep problems: We recruited 98 children with newly diagnosed NDD or follow-up on old diagnosis and found that there were 29 children with autism (29.6%), 17 children with ADHD (17.3%), 22 children with cerebral palsy (22.4%), 16 children with global developmental de- lay (16.3%) and 14 children with Intellectual disability (14.3%) (Table 4, Figures 4 and 5).
| ADHD | ASD | CP | GDD | ID | Total |
|---|---|---|---|---|---|
| 17 | 29 | 22 | 16 | 14 | 98 |
Table 4. Neurodevelopmental disorder.
It was observed that according to the cut-off validated SDSC, that is at 39, 73 children (74.5%) with a NDD showed a positive sleep score from the cut-off, while only 23 children (25.5%) were found to be below this value. In the control group, only 12 children (12.2%) had a positive sleep score, while 86 children (87.8%) were found to have a sleep score below the cut-off (Table 5).
| Positive Sleep Score | Status | |
|---|---|---|
| >39 | Control | Cases |
| No | 86 | 25 |
| Yes | 12 | 73 |
Table 5. Sleep disorder status cross tabulation.
We conducted Student’s t-test to determine if the differences in the sleep disorder in the control group and the NDD group were statistically significant. We found a p-value<0.01 and hence we can safely reject the null hypothesis and accept the alternate hypotheses that the NDD group had a statistically significant total sleep disorder score as compared to the control group (Table 6).
| Value | p-value |
|---|---|
| 77.3 | <0.001 |
Table 6. Chi-square test.
We also confirmed our findings using the Chi-square test to repeat similar results and we found that the p-value was less than 0.001 confirming our claim that sleep disorder is significantly different in cases and controls and that they are more common in children in NDD.
Specific sleep problems and patterns
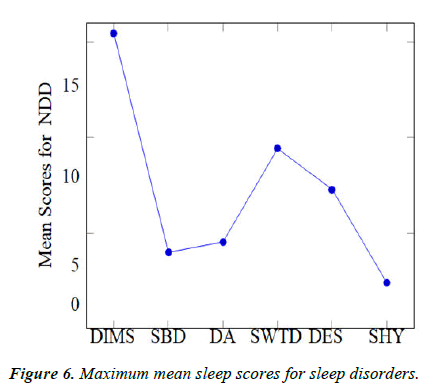
Sleep disorders grossly observed patterns: For the determination of our secondary objective, we aimed to compare the patterns of sleep disorders in the different neurodevelopmental disorders we had taken into account. These were namely, Disorder in Initiating and Maintaining Sleep (DIMS), Sleep Breathing Disorders (SBD), Disorders of Excessive Somnolence (DES), Sleep Wake Transition Disorder (SWTD), Disorders of Arousal (DA) and Sleep Hyperhydrosis (SHY) (Figure 6).
We calculated mean of the sleep scores of every Sleep disorder and compared it with each other. Data for the same is given in tables 7 and 8. Notably it was observed that:
| Sleep disorder | Max mean | NDD observed |
|---|---|---|
| DIMS | 15.471 | ADHD |
| SBD | 4 | CP |
| DA | 4.545 | CP |
| SWTD | 9.429 | ID |
| DES | 7.294 | ADHD |
| SHY | 2.412 | ADHD |
Table 7. Maximum mean scores for various sleep disorders.
| Sleep disorder | ASD | GDD | ADHD | CP | ID |
|---|---|---|---|---|---|
| DIMS | 12.345 | 12.063 | 15.471 | 15.091 | 12.143 |
| SBD | 3.276 | 3.313 | 3.824 | 4 | 3.286 |
| DA | 3.483 | 3.813 | 4 | 4.545 | 3.071 |
| SWTD | 8.931 | 7.5 | 7.412 | 8.818 | 9.429 |
| DES | 5.559 | 5.125 | 7.294 | 6.091 | 6.857 |
| SHY | 2.379 | 2.313 | 2.412 | 2.364 | 2.214 |
Table 8. Mean scores for various sleep disorders.
• For DIMS, a maximum mean score of 15.471, with respect to other NDD, was observed in children with ADHD (Table 7).
• For SBD, a maximum mean score of 4, with respect to other NDD, was observed in children with CP.
• For DA, a maximum mean score of 4.545, with respect to other NDD, was observed in children with CP.
• For SWTD, a maximum mean score of 9.429, with respect to other NDD, was observed in children with ID.
• For DES, a maximum mean score of 7.294, with respect to other NDD, was observed in children with ADHD.
• For SHY, a maximum mean score of 2.379, with respect to other NDD, was observed in children with ASD.
Through these tabulations, it was observed that the maximum sleep disorders were observed in children with ADHD, followed by children with CP (Figure 7).