Current Pediatric Research
International Journal of Pediatrics
Thiamine-responsive megaloblastic anemia syndrome: A rare case report
Samaher Jabril Ismail, Abdulmoein Eid Al-Agha*
King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
- Corresponding Author:
- Abdulmoein Eid Al-Agha
Professor, Pediatric Endocrinology, King Abdulaziz
University Hospital, Pediatric Department
PO Box: 80215 Jeddah 21589, Saudi Arabia
Tel: + 966 2 640 3841
Fax: + 966 2 6408353
E-mail: aagha@kau.edu.sa
Accepted date: October 09th, 2018
This case report describes a patient with a rare syndrome known as Thiamine-responsive Megaloblastic Anemia Syndrome (TRMA) which is an autosomal recessive disorder caused by gene mutation identified by megaloblastic anemia, progressive sensorineural hearing loss, and diabetes mellitus. We report a case of TRMA in a female child, born to a consanguine family. The child present with classical symptoms of the syndrome. Further assessment and mutation analysis confirmed a diagnosis of TRMA syndrome. By this report, we are hoping we can increase the awareness of the possible diagnosis of the syndrome for patients presenting with similar clinical manifestation especially where the rate of consanguineous marriages is high.
Keywords
Neonatal diabetes, Megaloblastic anemia, Deafness.
Introduction
Thiamine-responsive Megaloblastic Anemia Syndrome (TRMA) is a very rare syndrome defined by the following features: megaloblastic anemia, progressive sensorineural hearing loss, and variable onset monogenic diabetes mellitus [1]. Additionally, patients with TRMA syndrome may present with other disorders of the cardiovascular system such as congenital heart disease, arrhythmias, situs inversus viscerum, cardiomyopathy, stroke and other ophthalmological abnormalities in the retina, as well as damage to the optic nerve [2]. The symptoms of megaloblastic anemia manifest anytime between infancy and adolescence, although many patients present with symptoms in early childhood. Patients depend on lifelong oral replacement of thiamine [3]; however, they have macrocytic red blood cells despite receiving thiamine therapy, and megaloblastic anemia can manifest again after treatment cessation [1]. Sensorineural hearing loss is progressive, irreversible and diagnosed early in toddlers. Thiamine treatment, when started at an early stage (before two months old), may be effective in preventing deafness [4]. Patients have monogenic diabetes mellitus, which typically starts between infancy and adolescence. Evidence shows that treatment may delay diabetes onset and improve glucose tolerance [5,6]. We report the first case of TRMA in a three-year-old girl who was diagnosed after the development of neonatal diabetes mellitus, sensorineural deafness, and thiamine-dependent megaloblastic anemia.
Case Description
A three-year-old Saudi girl presented, at the age of four months, with pallor. She was the second child of healthy consanguineous (first cousin) parents. She was born full term, with a birth weight of 2.75 kg, through elective cesarean delivery. Her immunization, nutritional and developmental history was unremarkable, and her male sibling was healthy.
Laboratory investigations showed she was hyperglycemic, with a serum glucose level of 339 mg/ dL. A diagnosis of neonatal diabetes mellitus was made and she was treated with insulin. She was also found to be anemic and received repeated blood transfusions. Despite the blood transfusions, the patient was still anemic, and she was started on subcutaneous intermediate-acting insulin two to three times per day, according to her per-meal glucose reading.
Upon physical examination, the child looked normal and had no dysmorphic features. However, she had moderate to severe pallor with no signs of jaundice or cyanosis. She was active and had age-appropriate communication skills. Her vital signs were within the normal range, except for her heart rate, which was 115 beats per minute. Both her weight and height were in the 25th percentile. At the time of presentation, her complete blood count was as shown in Table 1.
| Test | First Presentation Before Treatment | After Confirming the Diagnosis and Starting Thiamine |
|---|---|---|
| Hemoglobin | 6.6 g/dL | 10 g/dL |
| Mean Corpuscular Volume | 83.8 fL | 73.8 fL |
| Hematocrit | 13.3% | 301% |
| Red blood cell count | 1.59 (106/µL) | 4.2 (106/µL) |
| White blood cell count | 14 (103/µL) | 10 (103/µL) |
| Platelet count | 132 (103/µL) | 576 (103/µL) |
| Ferritin level | 241.97 ng/mL | 246.97 ng/mL |
| Vitamin B1 (thiamine) | 3.5 μg/dL | 4.0 μg/dL |
Table 1. Laboratory findings at the patient’s initial presentation and after diagnosis
A peripheral blood smear showed normocytic hypochromic anemia with anisocytosis, polychromasia, poikilocytosis, spherocytes, thrombocytopenia, mild eosinophilia, and lymphocytosis. A renal profile, including sodium, potassium, calcium, blood urea nitrogen, creatinine, serum uric acid, phosphorus, and chloride was normal. A bone marrow biopsy was not done due to family refusal. Genetic evaluation for neonatal diabetes revealed a homozygous splice mutation in the SLC19A2 gene (c.1366-1G>c) at the conserved splice acceptor site of intron 5, and it is predicted to cause aberrant splicing. This result confirmed the genetic diagnosis of TRMA syndrome, and her parents were heterozygous for the SLC19A2 splicing mutation and carriers for the same mutation. An otoacoustic emission test was subsequently performed and severe bilateral sensorineural hearing loss was detected (80 dB HL). Hearing aids were therefore offered to the patient.
Discussion
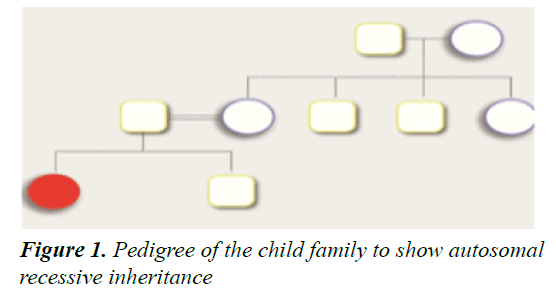
We present a novel mutation of SLC19A2 in a child with TRMA, which is a rare autosomal recessive disease with a high occurring rate among consanguineous families. Thiamine is an essential water-soluble vitamin that plays a fundamental role in many chemical reactions and cellular processes, including carbohydrate utilization and amino acid catabolism (Figure 1). Further, thiamine plays a role in neuronal signaling. Thiamine absorption occurs predominantly in the intestine, and its reabsorption from the renal tubules and other active cellular uptake is regulated by thiamine transporter (THTR-1), expressed by the SLC19A2 gene and THTR-2 expressed by the SLC19A3 gene. The three THTR-1 dependent cells, namely the β-cells of the pancreas, cochlear cells in the inner ear, and hematopoietic stem cells in the bone marrow, are the most affected in case of vitamin B1 deficiency [7-9]. A homozygous mutation in the SLC19A2 gene will lead to a decrease in intracellular thiamine in the THTR-1-dependent cells and result in the classical triad of symptoms of the syndrome megaloblastic anemia, monogenic diabetes mellitus, and sensorineural deafness [8]. A mutation in the gene will lead to the production of a non- functional thiamine transporter that will disrupt the active uptake of thiamine, resulting in thiamine deficiency. Cells in other body tissues have compensatory THTR-2 and will be less affected. On the other hand, thiamine shows a normal plasma range, implying that the THTR- 2 transporter is maintaining adequate absorption and keeping plasma thiamine within the normal range [10]. An adequate level of intracellular thiamine is necessary for normal pancreatic function, insulin secretion, and development of the auditory system [9-13].
Most children present with signs of anemia [1], which was the first observed symptom in our case. Pathological analyses of bone marrow samples in cases with TRMA typically show megaloblastic alterations, with erythroblasts usually having iron granule-filled mitochondria surrounding the nucleus (called sideroblasts) [3].
The anemia is classically corrected with thiamine treatment (Table 1). Leucopenia is less commonly seen in TRMA children, which explains the varying level of thiamine participation in each precursor of the hematopoietic stem cells [5]. In our case, the first clinical presentation was skin pallor, and a peripheral blood film showed normocytic hypochromic anemia with anisocytosis, polychromasia, poikilocytosis, and spherocytosis. The patient also had thrombocytopenia. In a previous case of homozygous SLC19A2 mutation in a diabetic patient with TRMA, a peripheral blood smear revealed macrocytic cells with no hemolytic findings [5]. In another case, the investigators did not find hematological changes typical of megaloblastic anemia [2]. Some investigators isolated cells from rats to assess the consequences of thiamine deficiency on the secretion of insulin; they showed that less insulin was secreted from the rats’ pancreatic islets cells [14-20]. Thiamineresponsive megaloblastic anemia syndrome is a good example of secondary diabetes due to a rare single gene disorder [5]. Many TRMA patients respond to vitamin treatment; however, others require insulin treatment, as was the case in our patient [9]. Other studies found that in the long-term, most cases become insulin-dependent by the time they reach puberty [12]. Selective loss of inner hair cells of the cochlea occurs due to thiamine deficiency caused by a mutation in the SLC19A2 gene.
This will result in irreversible sensorineural hearing loss, regardless of thiamine replacement therapy [3]. Unlike studies conducted on rats, thiamine can reverse deafness in human subjects [9]. In another case of TRMA in a onemonth old infant, hearing loss was not detected at the time of diagnosis and afterward [3,21].
Conclusion
Any child presenting with a triad of diabetes, hearing impairment and megaloblastic anemia should be suspected to have TRMA. A mutation analysis is warranted to detect the actual mutation and confirm the diagnosis. We believe that pre-symptomatic genetic testing may be helpful in detecting treatable conditions.
References
- Oishi K, Diaz GA. Thiamine-responsive megaloblastic anemia syndrome. GeneReviews 2003.
- Agladioglu SY, Aycan Z, Bas VN, et al. Thiamine-responsive megaloblastic anemia syndrome: A novel mutation. Genet Couns 2012; 23: 149-156.
- Mathews L, Narayanadas K, Sunil G. Thiamine responsive megaloblastic anemia. Indian pediatrics 2009; 46.
- Önal H, Barış S, Özdil M, et al. Thiamine-responsive megaloblastic anemia: early diagnosis may be effective in preventing deafness. The Turkish Journal of Pediatrics 2009; 51: 301-304.
- Tahir S, Leijssen LG, Sherif M, et al. A novel homozygous SLC19A2 mutation in a Portuguese patient with diabetes mellitus and thiamine-responsive megaloblastic anaemia. International Journal of Pediatric Endocrinology 2015; 1: 6.
- Franzese A, Fattorusso V, Mozzillo E. Thiamine-responsive megaloblastic anemia syndrome. InDiabetes associated with single gene defects and chromosomal abnormalities. Karger Publishers 2017; 25: 49-54.
- Diggireddy SR, Sindiri PR, Janampally R, et al. Thiamine responsive megaloblastic anemia in two female siblings. Journal of Evolution of Medical and Dental Sciences 2014; 3: 9989-9994.
- Villa V, Rivellese A, Di Salle F, et al. Acute ischemic stroke in a young woman with the thiamine-responsive megaloblastic anemia syndrome. The Journal of Clinical Endocrinology & Metabolism 2000; 85.
- Shaw‐Smith C, Flanagan SE, Patch AM, et al. Recessive SLC19A2 mutations are a cause of neonatal diabetes mellitus in thiamine‐responsive megaloblastic anaemia. Pediatric diabetes 2012; 13: 314.
- Reidling JC, Lambrecht N, Kassir M, et al. Impaired intestinal vitamin B1 (Thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 2010; 138: 1802-1809.
- Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochemistry International 1991; 25: 577-583.
- Ricketts CJ, Minton JA, Samuel J, et al. Thiamine‐responsive megaloblastic anaemia syndrome: Long‐term follow‐up and mutation analysis of seven families. Acta Paediatrica 2006; 95: 99-104.
- Shaw‐Smith C, Flanagan SE, Patch AM, et al. Recessive SLC19A2 mutations are a cause of neonatal diabetes mellitus in thiamine‐responsive megaloblastic anaemia. Pediatric Diabetes 2012; 13: 314.
- Agladioglu SY, Aycan Z, Bas VN, et al. Thiamine-responsive megaloblastic anemia syndrome: A novel mutation. Genet Couns 2012; 23: 149-156.
- Mathews L, Narayanadas K, Sunil G. Thiamine responsive megaloblastic anemia. Indian Pediatrics 2009; 46.
- Villa V, Rivellese A, Di Salle F, et al. Acute ischemic stroke in a young woman with the thiamine-responsive megaloblastic anemia syndrome. The Journal of Clinical Endocrinology & Metabolism 2000; 85.
- Franzese A, Fattorusso V, Mozzillo E. Thiamine-responsive megaloblastic anemia syndrome. InDiabetes associated with single gene defects and chromosomal abnormalities. Karger Publishers 2017; 25: 49-54.
- Diggireddy SR, Sindiri PR, Janampally R, et al. Thiamine responsive megaloblastic anemia in two female siblings. Journal of Evolution of Medical and Dental Sciences 2014; 3: 9989-9994.
- Reidling JC, Lambrecht N, Kassir M, et al. Impaired intestinal vitamin B1 (Thiamin) uptake in thiamin transporter-2–deficient mice. Gastroenterology 2010; 138: 1802-1809.
- Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochemistry International 1991; 25: 577-583.
- Ricketts CJ, Minton JA, Samuel J, et al. Thiamine‐responsive megaloblastic anaemia syndrome: Long‐term follow‐up and mutation analysis of seven families. Acta Paediatrica 2006; 95: 99-104.