Current Pediatric Research
International Journal of Pediatrics
A study on intravenous immunoglobulin with and without prednisolone in newly diagnosed pediatric primary immune thrombocytopenia.
Priyanka Gupta, Sumana Datta, Suprit Basu, Jyotirmoy Sen, Hasibur Rahaman Molla, Subham Bhattacharya*, Ahitagni Banerjee
Department of Pediatric Medicine, IPDMER and SSKM Hospital, Kolkata, India
- *Corresponding Author:
- Subham Bhattacharya
Department of Pediatric Medicine,
IPDMER and SSKM Hospital,
Kolkata, India
E-mail: drsubham@rediffmail.com
Received: 26 December, 2022, Manuscript No. AAJCP-23-85281; Editor assigned: 28 December, 2022, Pre QC No. AAJCP-23-85281(PQ); Reviewed: 05 January, 2023, QC No. AAJCP-23-85281; Revised: 20 January, 2023, Manuscript No. AAJCP-23-85281(R); Published: 31 January, 2023, DOI:10.35841/0971-9032.27.01.1765-1769.
Introduction: Immune Thrombocytopenic Purpura (ITP) is an immune-mediated disease characterized by a transient or persistent decrease in the platelet count. First-line agents for treatment include corticosteroids, Intravenous immunoglobulin (IVIG), and anti-D immunoglobulin (anti-D). This study was done to compare the efficacy of IVIG-prednisolone combination therapy with IVIG monotherapy in achieving sustained complete response to newly diagnosed childhood ITP.
Methodology: This was a prospective observational study conducted in the department of pediatric medicine in a tertiary care super specialty hospital in Eastern India. Children between 6 months to 12 years with newly diagnosed primary ITP with bleeding and/or platelet count <20,000/cmm were included in the study. The study population was divided into two cohorts: One cohort received IVIG at 0.8 g/kg single dose alone (IVIG cohort) and another one received IVIG in the same dose along with prednisolone 2 mg/kg/day for 14 days followed by quick tapering, maximally over 2 weeks. Both cohorts were followed up for 6 months.
Results: Complete Response (CR) was significantly higher in the IVIG-prednisolone cohort, compared to the IVIG cohort. Also, children in IVIG -prednisolone cohort maintained substantially higher median platelet count. The adverse effects in both cohorts were mild and self-limiting.
Conclusion: IVIG along with prednisolone as first-line treatment in newly diagnosed pediatric ITP is more efficacious in achieving sustained response and thus, decreasing the chronicity of the disease compared to IVIG monotherapy.
Keywords
IVIG, Prednisolone, Immune thrombocytopenic purpura, Pediatric.
Introduction
Immune Thrombocytopenia (ITP) is an immune-mediated acquired disease of adults and children characterized by a transient or persistent decrease in the platelet count [1]. ITP may be primary or secondary [2]. Primary ITP is an autoimmune disorder characterized by isolated thrombocytopenia (platelet count<100 × 109/L) in the absence of other causes or disorders. The diagnosis of primary ITP remains one of exclusion [3,4]. The peak incidence is in children 2 to 5 years of age. Newly diagnosed ITP is defined as up to 3 months from diagnosis, persistent ITP is 3–12 months from diagnosis, and chronic is defined as ITP lasting more than 12 months [5]. The recent ASH guideline suggests steroids, IVIG, and anti-D as first-line treatment options [6]. Despite the best efforts of treatment, around one-third of patients with newly diagnosed ITP fail to respond to steroids or IVIG or lose the initially attained response and become persistent or chronic ITP [7,8].
The rationale for using combination therapy like IVIG along with steroids is to use agents with non-overlapping toxicities to target different pathways involved in autoantibody production and platelet destruction. Various studies combine IVIG with steroids and attain better response rates. Most of these studies have been done in the adult age group [9-12]. Such studies in the pediatric age group are largely lacking. Hence the study was done to evaluate the role of IVIG along with prednisolone (IVIG-Prednisolone) in achieving a higher and sustained rate of complete response in newly diagnosed primary pediatric ITP and compare it to those receiving IVIG monotherapy.
Materials and Methods
This was a prospective observational study conducted in the department of pediatric medicine in a tertiary care super specialty hospital in eastern India. Children between 6 months to 12 years with newly diagnosed primary ITP with bleeding and/or platelet count<20,000/cmm were included in the study. Patients who were having secondary ITP; cardiac, pulmonary, hepatic, or renal dysfunction; had taken antiplatelet or Non- Steroidal Anti-Inflammatory Drugs (NSAIDS) within one month from the commencement of the study, or had other bleeding disorders or coagulopathy, were excluded from the study.
After obtaining the demographic data, detailed history, examination, grades of bleed, baseline platelet count, and other laboratory investigations to confirm the diagnosis of newly diagnosed ITP, patients were divided into two comparable cohorts. One cohort was treated with IVIG (0.8 g/kg/day) single dose, over 24-48 hours, and the second cohort with IVIG along with prednisolone IVIG (0.8 g/kg/day) single dose with tab prednisolone at 2 mg/kg/day for 14 days starting from D1, followed by quick tapering, maximally over 2 weeks. Patients were entitled to get any rescue therapy in case of any major bleeding.
The sample size was calculated based on the formula:
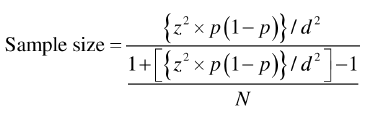
Z=Value of standard normal distribution=1.96,
p=Proportion in infinity population,
q=(1-p),
d=Absolute precision-0.04,
N=Average no. of ITP patients admitted to our institution,
We got a sample size of approximately 52.
Patients were enrolled in this study from 1st February 2019 to 31st July 2020 (18 months). As a sustained response, denominated as the overall response in this study, patients were followed up to January 2021, i.e., minimal of 6 months. Data were taken regarding platelet counts, bleeding symptoms, and adverse effects. The response to treatment was evaluated according to International Working Group (IWG) criteria [13,14].
The primary endpoint defined in this study was to evaluate the incidence of attainment of Complete Response (CR) (platelet count >100 × 109/L and no bleeding) at the end of 6 months. The secondary endpoint is defined as a recurrence of thrombocytopenia and maintenance of a sustained higher median platelet count. Platelet count was done initially biweekly followed by on monthly basis. Bleeding grades were assessed at baseline followed by a monthly interval.
All procedures performed in studies involving human participants were by the ethical standards of the institutional and national research committee with the 1964 declaration of Helsinki and its later amendments or comparable ethical standards. The study obtained ethical clearance from institutional ethical committee.
Statistical analysis: Categorical variables are expressed as the number of patients and percentage of patients and compared across the groups using Pearson’s chi-square test/fisher's exact test as appropriate. Continuous variables are expressed as mean, median, and standard deviation and compared across the groups using the Mann-Whitney U-test. SPSS version 20 has been used for the analysis and any p-value less than 0.05 has been considered significant.
Results
A total of 50 newly diagnosed Immune thrombocytopenia patients were enrolled in the study, 25 patients each in IVIG and IVIG-Prednisolone cohort. No patients died or exit the study. In our study, the patients presented mainly with grade 1 (58%) followed by grade 2 (40%) of bleeding. Only 2% of the subjects had a severe bleed, grade 3. The mean baseline platelet count at presentation in the IVIG cohort was 19080.00± 15190.24/cmm and in IVIG and prednisolone cohort it was 16920.00 ± 8939.24/cmm.
There is no significant difference in age or sex distribution between the two cohorts (Table 1). Maximum cases were reported in March followed by November. Only 26% of total patients had a history of preceding viral infection. The duration of the disease and baseline average platelet count before the intervention are confounding factors that may lead to misinterpretation of results therefore, both these entities were compared statistically, and there was no significant difference (Table 2). Patients were divided into two cohorts, which were comparable before therapy in epidemiological, clinical and hematological aspects.
| Cohort | Total | p-value | Significance | |||
|---|---|---|---|---|---|---|
| IVIG | IVIG+Prednisolone | |||||
| AGE | ≤1 Year | 5 (20%) | 1 (4%) | 6 (12%) | 0.55 | Not Significant |
| 1-2 Years | 2 (8%) | 2 (8%) | 4 (8%) | |||
| 2-3 Years | 3 (12%) | 3 (12%) | 6 (12%) | |||
| 3-4 Years | 0 (0%) | 1 (4%) | 1 (2%) | |||
| 4-5 Years | 4 (16%) | 6 (24%) | 10 20%) | |||
| >5 Years | 11 44%) | 12 (48%) | 23 46%) | |||
| Gender | Female | 9 (36%) | 10 (40%) | 19 38%) | 0.771 | Not Significant |
| Male | 16 64%) | 15 (60%) | 31 62%) | |||
Table 1. Distribution of age and gender in the study population.
| Cohort | p-value | Significance | ||||||
|---|---|---|---|---|---|---|---|---|
| IVIG (n=25) | IVIG+Prednisolone (n=25) | |||||||
| Mean | Median (IQ-Range) | Std. Deviation | Mean | Median (IQ-Range) | Std. Deviation | |||
| Onset of bleed(days) | 17.64 | 6 (3.5-10) | 37.16 | 60.68 | 5 (3-14) | 173.99 | 0.891 | Not Significant |
| Platelets (per cmm) | 19080 | 15000 (10000-20000) | 15190.24 | 16920 | 15000 (10000-22500) | 8939.24 | 0.976 | Not Significant |
Table 2. Comparison of duration of disease and baseline platelet in both the cohorts.
The total no of patients achieving CR was significantly higher in the IVIG-prednisolone cohort compared to IVIG. Also, uniform non-response (not achieved CR at any point in the study) and recurrence of thrombocytopenia were higher in the IVIG cohort compared to another one, but the difference is not statistically significant probably because of the smaller sample size (Table 3). The median time to respond was similar in both cohorts.
| Characteristics | IVIG (n=25)) (%) | IVIG + Prednisolone (n=25) (%) | Significance test | p-value | Significance |
|---|---|---|---|---|---|
| Complete Response (CR) | 11 (44%) | 19 (76%) | Pearson’s chi-square test for independence of attributes | 0.021 | Significant |
| Uniform non- response | 8 (32%) | 4 (16%) | Pearson’s chi-square test for independence of attributes | 0.185 | Not significant |
| Recurrence of thrombocytopenia | 6 (24%) | 2 (8%) | Fisher's exact test | 0.247 | Not significant |
| Median time (months) (IQR) | 1 (1-1) | 1 (1-1) | Mann Whitney-U-test | 0.476 | Not significant |
Table 3. Outcome at the study closure (6 months).
During follow-up, children in the IVIG-prednisolone cohort persistently maintained the CR as compared to the IVIG cohort, which was statistically significant. At the end of 6 months, it was seen that in the IVIG cohort, 24% of the patient developed recurrence of thrombocytopenia while it was 8% in IVIGprednisolone cohort (Table 4).
| Follow up | IVIG cohort (n=25) | IVIG and prednisolone (n=25) | p-value | Significance |
|---|---|---|---|---|
| 1st Month | 15 (60%) | 21 (84%) | 0.059 | Not significant |
| 2nd Month | 12 (48%) | 21 (84%) | 0.007 | Significant |
| 3rd Month | 11 (44%) | 22 (88) | 0.001 | Significant |
| 4th Month | 12 (48%) | 21 (84%) | 0.007 | Significant |
| 5th Month | 11 (44) | 20 (80) | 0.009 | Significant |
| 6th Month | 11 (44%) | 19 (76%) | 0.021 | Significant |
Table 4. Follow-up: monthly comparison of patients attaining CR in both cohorts.
The median platelet count was always higher and sustained in the IVIG-Prednisolone cohort during each follow-up, and it was significantly higher during the 3rd and 4th months of treatment (Table 5 and Figure 1). The adverse effects on both the cohorts were mild and self-limiting and no statistically significant difference was noted between the two cohorts (Table 6).
| IVIG median (IQ range) | IVIG+prednisolone median (IQ range) | p-value | Significance | |
|---|---|---|---|---|
| 1st Month follow up | 120000 (46500-245000) | 160000 (110000-232000) | 0.846 | Not significant |
| 2nd Month follow up | 80000 (44000-230000) | 180000 (120000-230000) | 0.105 | Not significant |
| 3rd Month follow up | 60000 (30000-220000) | 160000 (145000-270000) | 0.021 | Significant |
| 4th Month follow up | 60000 (28000-250000) | 220000 (120000-290000) | 0.011 | Significant |
| 5th Month follow up | 42000 (28000-265000) | 200000 (127000-275000) | 0.137 | Not significant |
| 6th Month follow up | 45000 (29000-270000) | 210000 (90000-310000) | 0.187 | Not significant |
Table 5. Comparison of median platelet count between the two cohorts..
| IVIG (n=25) No (%) | CTC grade (no of patients) | IVIG and prednisolone (n=25) No (%) | CTC grade (no of patients) | p-value | Significance | |
|---|---|---|---|---|---|---|
| Headache | 3 (12%) | I | 0 | 0.235 | Not Significant | |
| Fever | 3 (12%) | I | 2 (8%) | I | 0.637 | Not Significant |
| Fatigue | 1 (4%) | I | 0 | 0.312 | Not Significant | |
| Nausea | 3 (12%) | I (2) II (1) |
5 (20%) | I (3) | 0.702 | Not Significant |
| Vomiting | 0 | 2 (8%) | I | 0.49 | Not Significant | |
| Gastritis | 1 (4%) | I | 4 (16%) | I (1) II (3) |
0.349 | Not Significant |
| Weight gain | 0 | 1 (4%) | I | - | ||
| Hypertension | 0 | 0 | - | |||
| Hyper glycaemia | 0 | 0 | - | |||
| Anaphylaxis | 0 | 0 | - | |||
| Bronchospasm | 0 | 0 | - | |||
| Mood changes | 0 | 0 | - |
Table 6. Adverse effects with their CTC grading.
Discussion
In our study we have compared treatment outcomes of combination therapy (IVIG and prednisolone) with IVIG monotherapy in newly diagnosed ITP children aged 6 months to 12 years in terms of incidence of attainment of CR, recurrence of thrombocytopenia, maintenance of a sustained higher median platelet count and adverse effects. Both cohorts were comparable before therapy in epidemiological, clinical and hematological aspects and a similar brand of IVIG (plasmaglob 5%) was used for all patients.
At the end of this study, it was seen that in patients receiving IVIG-prednisolone, complete response was achieved 76% of children compared to 44% with IVIG monotherapy with a pvalue of 0.021. This finding was supported by a study done by Parodi et al. [15] on 34 children with immune thrombocytopenia.
Also, we calculated the incidence of persistent ITP at the end of 6 months and it was seen that the incidence of persistent ITP was higher (56%) in patients receiving IVIG monotherapy compared to IVIG-prednisolone (24%). A study done by Eshagh-Hosseini et al. [16] showed a similar result. Further efficacy of combination therapy is supported by studies done by Greige et al. [17] in the year 2000 and Blanchette et al. [18].
It was also noted that the median platelet count was significantly higher in those receiving combination therapy than receiving IVIG monotherapy. Similar results were obtained by a study done by Godeau et al. in adults. There was no significant relationship between the occurrences of adverse effects in both cohorts (p-value=>0.05).
Conclusion
From this study, it was concluded that IVIG along with prednisolone as first-line treatment in newly diagnosed pediatric ITP is more efficacious, compared to IVIG monotherapy, in achieving sustained response, decreasing the chronicity of the disease, and maintaining a higher and sustained median platelet count. There is also a lesser incidence of recurrent thrombocytopenia with IVIGprednisolone combination therapy with no added adverse effect. The study is the first of its kind to compare specifically IVIG with IVIG-Prednisolone combination therapy in newly diagnosed pediatric ITP. However, this present study was limited by a single centric small sample size and a shorter duration of follow-up (6 months).
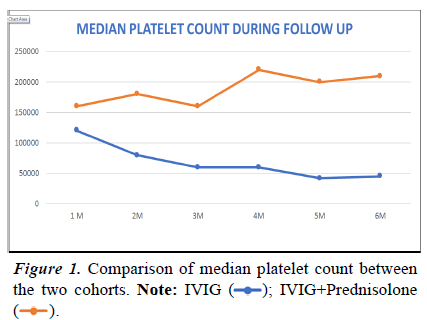
 ); IVIG+Prednisolone (
); IVIG+Prednisolone ( ).
).