Current Pediatric Research
International Journal of Pediatrics
Mother to child transmission rate of hepatitis B after tenofovir disoproxil fumarate implementation.
Sarawut Boonsuk1,2*, Weerawan Hattasingh1, Kriengsak Limkittikul1, Prakaykaew Charunwatthana3, Kulkanya Chokephaibulkit4, Ngamphol Soonthornworasiri5, Chaweewon Boonshuyar6
1Department of Tropical Pediatrics, College of Tropical Medicine, Mahidol Universit y, Nakhon Pathom, Thailand
2Department of Health Ministry of public health, College of Tropical Medicine, Mahidol University, Nakhon Pathom, Thailand
3Department of Clinical Tropical Medicine, College of Tropical Medicine, Mahidol University, Nakhon Pathom, Thailand
4Department of Infectious Diseases, Siriraj Hopsital, Mahidol University, Nakhon Pathom, Thailand
5Department of Tropical Hygiene, College of Tropical Medicine, Mahidol University, Nakhon Pathom, Thailand
6Department of Public Health, Thammasat University, Phra Nakhon, Thailand
- *Corresponding Author:
- Sarawut Boonsuk
Department of Tropical Pediatrics Faculty of Tropical Medicine Mahidol University Nakhon Pathom,
Thailand,
E-mail: Wutmd39ju@hotmail.com
Received: 28 June, 2022, Manuscript No. AAJCP-22-65697; Editor assigned: 30 June, 2022, PreQC No. AAJCP-22-65697 (PQ); Reviewed: 13 July, 2022, QC No. AAJCP-22-65697; Revised: 17 July, 2022, Manuscript No. AAJCP-22-65697 (R); Published: 29 July, 2022, DOI:10.35841/0971-9032.26.8.1539-1545.
Hepatitis B virus remains a major public health problem around the world, especially in developing countries in Asia prevalence more than 8%. In Thailand, there are thousands of children under the age of 5 years have HBV infected from mother to child transmission. Infants infected with HBV from their mothers are at risk of developing hepatocellular carcinoma and hepatic cancer at 90%. Since 2017, Thai national guidelines have recommended that mothers with high viral load or HBeAg positivity use Tenofovir Disoproxil Fumarate (TDF) to prevent HBV transmission from mother to child. This study armed to evaluate of the transmission rate and factors associated with Mother to Child Transmission (MTCT). The retrospective cohort studies analysis was conducted using the transmission of hepatitis B from mother to child data. Records of 342 pregnancy woman who hepatitis B infection were obtained. Participants mother at study entry must be Hepatitis B Surface Antigen (HBsAg) positive from 2018 to 2020 were reviewed. Details of the mother factors such as Underlying disease, Fertility planning, ANC visit, HBeAg status, viral load level and mode of delivery and Infant factors such as body weight, active- passive immunoglobulin, breast milk status included TDF used for mother HBV prevention data. Transmission rate was calculated and risk factors for MTCT were assessed using a multivariable binary regression model. There were 42.40% (145) infants born from mothers received TDF and 57.60% (197) infants born to non TDF-used mothers. 52.92% were uninformed that they had hepatitis B, and more than half (52.34%) were diagnosed as hepatitis B positive during their pregnancies. All 342 infants received hepatitis B vaccine at birth, 323 infants received Hepatitis B Immune Globulin (HBIG) and hepatitis B vaccine and 5.56% (19) who did not received Hepatitis B Immune Globulin (HBIG). The overall MTCT incidence rate is 0.88%, the MTCT rate among TDF mothers is 0.69%, and the MTCT rate among non-TDF mothers is 1.02%. However no association between risk factor and MTCT among mothers HBsAg positive.
Keywords
Mother to child transmission, Hepatitis B, Tenofovir disoproxil fumarate, Risk factors.
Introduction
Hepatitis B virus remains a major public health problem around the world, an estimated 2 billion people, or 30% of the population infected with hepatitis B virus [1] In each year, about 600,000 people died from liver cancer, cirrhosis and liver failure related to hepatitis B [2,3]. According to previous research, infant have a 90% chance of becoming chronic carriers after HBV infection, and children under the age of three have a 50% chance [4,5]. In Thailand the rate of HBV infection among women who pregnancy is stills at a high a rate of 11.19 cases per 100,000 people [6]. Since 2017, Thai national guidelines have recommended that mothers with high viral load or HBeAg positivity use Tenofovir Disoproxil Fumarate (TDF) to prevent HBV transmission from mother to child.
In Thailand, thousands of children under the age of five are infected with HBV from their mothers, resulting in a 0.1 prevalence of HBV infection [7]. Hepatitis B virus transmission from mother to child is thus a cause of chronic hepatitis. Infants infected with HBV from their mothers have a 90% chance of developing hepatocellular carcinoma and hepatic cancer. According to the information, the Hepatitis B virus is transmitted from mother to child. In Thailand, it is a major cause of chronic hepatitis. Nonetheless, few studies have been conducted to establish the transmission rate and factors associated with the transmission of hepatitis B from mother to child following the implementation of tenofovir disoproxil fumarate in Thailand.
Methods
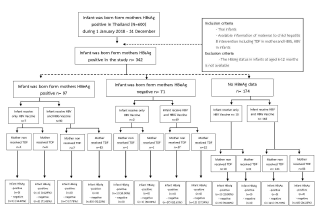
The retrospective cohort study in Thai hospitals aimed to evaluate the transmission rate and factors associated with Mother-To-Child Transmission (MTCT). This study included 342 pregnant women who were diagnosed with Hepatitis B Surface Antigen (HBsAg) positivity between 2018 and 2020. We assessed Hepatitis B virus transmission from mother to child among mothers who used Tenofovir Disoproxil Fumarate (TDF) and those who did not use Tenofovir Disoproxil Fumarate (TDF). Hepatitis B Surface Antigen (HBsAg) positivity in pregnant women and infants born from Hepatitis B Surface Antigen (HBsAg) positive mothers were the inclusion criteria for this study. Data was collected and recorded from hospitals throughout Thailand using a case record form.
Nurses and doctors complete all case record forms. The case record form collected information on the mother's general characteristics, details of her hepatitis B infection, TDF treatment and untreated, and mode of delivery; the infant's information included general characteristics, vaccination status, and breast milk status. All statistical analysis was carried out using Stata software version 20. The significance tests were two-sided, and p-values less than 0.05 were considered to be statistically significant. All statistical analysis was carried out using Stata software version 20. The significance tests were two-sided, and p-values less than 0.05 were considered to be statistically significant. For categorical variables, we used chi-square and fisher's exact tests. Using univariate and multivariate analysis, examine factors associated with hepatitis B transmission from mother to child. The study was conducted in accordance with the standards and was approved by the Ministry of Public Health (MOPH) ethical committee No.505/2564.
Results
A total of 342 pregnant women with Hepatitis B Surface Antigen (HBsAg) positivity were included in the study cohort. Pregnant women's data is gathered from hospitals throughout Thailand. All patients had previously been screened for HBV, according to Thailand's national guidelines for caring for pregnant women and children to prevent the transmission of Hepatitis B virus from mother to child.
Table 1 includes data about the mother's general characteristics, her hepatitis B infection, TDF treatment and untreated, and mode of delivery; the infant's information includes general characteristics, vaccination status, and breast milk status. Based on 197 (57.60%) mothers none receive Tenofovir Disoproxil Fumarate (TDF) and 145 (42.40%) mothers receive Tenofovir Disoproxil Fumarate (TDF). The mothers' general characteristics were that the median age of hepatitis B infected mothers was 30 years old, with 46.20. There was no underlying disease in 84.21% of the mothers. When they became pregnant 47.66% of women had been diagnosed with hepatitis B. HBeAg positivity was found in 28.36% of the population. At the same time, 22 mothers (6.43%) had a viral load greater than or equal to 200,000 IU/ml before receiving TDF, 55.17% had received TDF more than or equal to four weeks before giving birth, and 34.80% had not arranged family planning. The majority of them (61.11%) went to antenatal care more than five times during their pregnancy, and the majority had normal labor (61.99%).
Females made up the majority of infants born to hepatitis B mothers, accounting for 51.75% of all births. More than half of the infants (63.74%) were breastfed. They were all given the HBV vaccine. Only 19 infants did not receive Hepatitis B Immune Globulin (HBIG).
Risk factors for HBV mother to child transmission are shown in Table 2. The risk factors and mother-to-child transmission were studied using univariate binary regression analysis. However, no associations were found between maternal factors, infant factors, and mother-to-child transmission with a statistical significance level of 0.05. According to Table 3, no factors are associated to mother-to-child transmission in infants born to HBV-infected mothers using multivariate binary regression (Tables 1-3).
| Characteristics | Mothers non receive TDF | Mothers receive TDF | Total (%) | P-value | |||
|---|---|---|---|---|---|---|---|
| Number (%), n=197 (57.60) | Number (%), n=145 (42.40) | Number (%), n=342 (100) | |||||
| Maternal factor | |||||||
| Age (Years) (Median=30, Max=44, Min=14) | |||||||
| <20 | 5 (2.54) | 5 (3.45) | 10 (2.92) | 0.836 | |||
| 21-30 | 101 (51.27) | 71 (48.97) | 172 (50.29) | ||||
| >31 | 91 (46.19) | 69 (47.59) | 160 (46.78) | ||||
| Underlying disease | |||||||
| Yes | 31 (15.74) | 23 (15.86) | 54 (15.79) | 0.545 | |||
| No | 166 (84.26) | 122 (84.14) | 288 (84.21) | ||||
| Diagnose HBV | |||||||
| Before pregnancy | 99 (50.25) | 64 (44.14) | 163 (47.66) | 0.263 | |||
| During pregnancy | 98 (49.75) | 81 (55.86) | 179 (52.34) | ||||
| HBeAg | |||||||
| No test | 131 (66.50) | 43 (29.66) | 174 (50.88) | <0.001* | |||
| HBeAg negative | 58 (29.44) | 13 (8.97) | 71 (20.76) | ||||
| HBeAg positive | 8 (4.06) | 89 (61.38) | 97 (28.36) | ||||
| Viral load before TDF (IU/ml) | |||||||
| No test | 195 (98.98) | 118 (81.38) | 313 (91.52) | <0.001a* | |||
| <2000,000 | 0 | 7 (4.38) | 7 (2.05) | ||||
| ≥ 200,000 | 2 (1.02) | 20 (13.79) | 22 (6.43) | ||||
| Adherence TDF (n=145) | |||||||
| <4 weeks | 0 | 65 (44.83) | 65 (44.83) | † | |||
| ≥ 4 weeks | 0 | 80(55.17) | 80 (55.17) | ||||
| Family planning | |||||||
| No | 73 (37.06) | 46 (31.72) | 119 (34.80) | 0.182 | |||
| Yes | 124 (62.94) | 99 (68.28) | 223 (65.20) | ||||
| ANC visit | |||||||
| 1- 4 times | 80 (40.61) | 53 (36.55) | 133 (38.89) | 0.447 | |||
| ≥ 5 times | 117 (59.39) | 92 (63.45) | 209 (61.11) | ||||
| Mode of delivery | |||||||
| Normal labor | 119 (60.41) | 93 (64.14) | 212 (61.99) | 0.003 a* | |||
| Caesarean section | 65 (32.99) | 52 (35.86) | 117 (34.21) | ||||
| Forceps delivery and vacuum extraction | 13 (6.60) | 0 | 13 (3.80) | ||||
| infant factor | |||||||
| Gender | |||||||
| Male | 99 (50.25) | 66 (45.52) | 165 (48.25) | 0.386 | |||
| Female | 98 (49.75) | 79 (54.48) | 177 (51.75) | ||||
| Breast feeding | |||||||
| No | 85 (43.15) | 39 (26.90) | 124 (36.26) | 0.001* | |||
| Yes | 112 (56.85) | 106 (73.10) | 218 (63.74) | ||||
| HBV vaccine | |||||||
| Yes | 145 (100) | 197 (100) | 342 (100) | † | |||
| HBIG vaccine | |||||||
| No | 12 (6.09) | 7 (4.83) | 19 (5.56) | 0.614 | |||
| Yes | 185 (93.91) | 138 (95.17) | 323 (94.44) | ||||
| HBV and HBIG | |||||||
| No | 12 (6.09) | 7 (4.83) | 19 (5.56) | 0.614 | |||
| Yes | 185 (93.91) | 138 (95.17) | 323 (94.44) | ||||
Table 1. Characteristics of maternal and infant. *: Significance of level 0.05 a fisher exact test, †: The p-value was not available.
| Factors | Infant uninfected | Infant infected | RR (95%CI) | P-value | |
|---|---|---|---|---|---|
| Mothers factor | |||||
| TDF | |||||
| No | 195 (98.98) | 2 (1.02) | 0.679 (0.062 – 7.539) | 0.751 | |
| Yes | 144 (99.31) | 1 (0.69) | |||
| Diagnose HBV | |||||
| Before pregnancy | 163 (100) | 0 | † | ||
| During pregnancy | 176 (98.32) | 3 (1.68) | |||
| HBeAg | |||||
| No test | 173 (99.43) | 1 (0.57) | † | ||
| HBeAg Positive | 70 (98.97) | 1 (1.03) | |||
| HBeAg negative | 96 (98.59) | 1 (1.41) | |||
| Viral load before TDF (IU/ml) | |||||
| No test | 311 (99.36) | 2 (0.64) | † | ||
| <2000,000 | 7 (100) | 0 | |||
| ≥ 200,000 | 21 (95.45) | 1 (4.55) | |||
| Family planning | |||||
| No | 117 (98.32) | 2 (1.68) | 0.267 (0.024 – 2.912) | 0.279 | |
| Yes | 222 (99.55) | 1 (0.45) | |||
| Gestational | |||||
| 1-3 | 248 (99.20) | 2 (0.80) | 1.358 (0.124-14.800) | 0.08 | |
| ≥ 4 | 91 (98.91) | 1 (1.09) | |||
| Gestational age at birth | |||||
| <37 Weeks | 291 (99.32) | 2 (0.68) | 0.334 (0.309–3.618) | 0.367 | |
| ≥ 37 Weeks | 48 (97.96) | 1 (2.04) | |||
| Mode of delivery | |||||
| Vaginal labor | 225 (100) | 0 | † | ||
| Caesarean section | 114 (97.44) | 3 (2.56) | |||
| Infant factor | |||||
| Male | 164 (99.39) | 1 (0.61) | 0.628 (0.049–5.860) | 0.609 | |
| Female | 175 (98.39) | 2 (1.13) | |||
| Breast feeding | |||||
| No | 124 (100) | 0 | † | ||
| Yes | 215 (98.62) | 3 (1.38) | |||
| Only HBV vaccine | |||||
| No | 323 (100) | 0 | † | ||
| Yes | 16 (84.21) | 3 (15.79) | |||
| HBV and HBIG | |||||
| No | 16 (84.21) | 3 (15.79) | † | ||
| Yes | 323 (100) | 0 | |||
Table 2. Univariate binary regression analysis of risk factor for HBV mother to child transmission. *: Significance of level 0.05, †: The relative risk was not available.
| Factors | RR (95%CI) | p-value |
|---|---|---|
| TDF | 0.230 (0.008 – 6.315) | 0.385 |
| Yes | ||
| No | ||
| HBeAg | 2.698 (0.414 – 17.561) | 0.299 |
| No test | ||
| HBeAg positive | ||
| HBeAg negative | ||
| Family planning | 0.194 (0.015 – 2.478) | 0.208 |
| Yes | ||
| No | ||
| Gestational number | 1.303 (0.121 – 13.940) | 0.827 |
| 1-3 | ||
| ≥ 4 | ||
| Gestational age at birth | 0.232 (0.021-2.561) | 0.234 |
| ≥ 37 Weeks | ||
| <37 Weeks | ||
| Gender (Infant) | 2.144 (0.195-23.554) | 0.533 |
| Male | ||
| Female |
Table 3. Multivariate binary regression of relative risk for mother to child transmission.
Discussion
The findings were consistent with other studies that found a difference in the mother-to-child transmission rate among TDF-using mothers. For example, a study of mothers with HBV DNA levels greater than 200,000 IU/ml during the third trimester discovered that TDF-taking mothers had a lower mother-to-child transmission rate than non-TDF-taking mothers [8]. The findings were also consistent with previous research on the rate of hepatitis B transmission from mother to child after TDF treatment. In a Chinese study, mothers with a high viral load had a 0.7 mother-to-child transmission rate of hepatitis B after receiving TDF [9]. It was related to the study by Calvin Q, who conducted a randomized controlled trial to compare TDF-taking and non-TDF-taking mothers and found that the TDF-taking group had a lower mother-to-child transmission rate than the non-TDF-taking group with statistical significance [10]. Furthermore, the findings were consistent with a meta-analysis of 1578 articles, which found that taking TDF during pregnancy reduced the risk of hepatitis B infection in infants born to hepatitis B mothers and was effective in preventing hepatitis B infection, particularly in those resistant to lamivudine [11]. Gonzague's RCT study in Thailand with 328 mothers from 28 weeks to two months postpartum observed that the mother-to-child transmission rate of hepatitis B after receiving TDF was 3%, whereas the placebo group had a 6% rate. As a result, there was no statistically significant difference. Finally, a Chinese study on the efficacy of TDF in preventing mother-to-child transmission discovered that TDF-using mothers could effectively reduce mother-to-child transmission [12].
Receiving HBIG and HBV vaccines was consistent with previous studies, such as one that established that receiving HBIG and HBV vaccines within 1 hour of birth could prevent mother-to-child transmission of hepatitis B. It was consistent with a Panpan study, which discovered that providing HBIG and HBV vaccines within 12 hours of birth could reduce mother-to-child transmission by 90% [4]. This was similar to the study's finding that no infant who received HBIG and HBV vaccines within 12 hours of birth became infected through mother-to-child transmission. A study conducted in the United States of America found that giving HBIG and HBV vaccines to high-risk infants reduced mother-to-child transmission better than receiving plasma-derived vaccine [13], which agreed with a study by Zhe, who conducted a systematic review study and found that having given HBIG and HBV vaccines to pregnant women who tested positive for HBsAg could prevent mother-to-child transmission of hepatitis B [14]. Furthermore, a study comparing HBIG 100 IU and 200 IU doses among hepatitis B mothers discovered that using HBIG 100 IU at birth could prevent mother-to-child transmission of hepatitis B [15].
In this study, the efficacy of TDF was 32%. The TDF-taking group had a lower mother-to-child transmission rate than the non-TDF-taking group (1.02 vs 0.69) relative risk is 0.679, which was consistent with other studies that found TDF to be effective in preventing MTC. Furthermore, there were no reports of TDF side effects in pregnant women [16]. The findings were also consistent with a study conducted in the United Kingdom, which identified that long-term use of TDF had a long-term positive effect among the chronic HBV group, and there was no liver fibrosis or abnormal kidney function in the group that received TDF for 48 weeks [17].
Conclusion
From the data collection of 342 mothers with HBsAg positive from 2018 to 2020 in Thailand, the overall mother-to-child transmission rate of hepatitis B was 0.88%, and the mother-to-child transmission rate of hepatitis B after receiving TDF was 0.69%. The infection rate among non TDF-taking mothers was 1.02%, and mothers with HBeAg positivity were 1.03%.
However, there was no statistically significant difference between the relationship test and the relative risk of hepatitis B transmission from mother to child and other factors, which was consistent with the multivariate binary regression relative test, which also found no statistically significant difference. Mothers with HBeAg positive group, one infant (14.29%) got hepatitis B from mother-to-child transmission among the TDF-taking mothers and the infants who received only HBV vaccine. In the group of the mothers with HBeAg negative, the result found that one infant (50.00%) got hepatitis B from mother-to-child transmission among the non TDF-taking mothers and the infants who received only HBV vaccine. In the group of the mothers with no HBeAg data, the result found that one infant (10.00%) got hepatitis B from mother-to-child transmission among the non TDF-taking mothers and the infants who got only HBV vaccine. When analyzed the mother-to-child transmission by vaccination, there were groups of infants who received only HBV vaccine and infants who received HBIG (Hepatitis B Immune Globulin) and HBV vaccine. The result found that there was no mother-to-child transmission among infants who received HBIG and HBV vaccine, according to the summary flowchart (Figure 1).
Recommendations
- Before 12 weeks of gestation, pregnant women should receive antenatal care.
- TDF should be given to pregnant women who have HBsAg, HBeAg, or a viral load of 200,000 IU/ml.
- The viral load should not be tested after less than 24 weeks of TDF treatment.
- Pregnant women in high-risk groups must be given TDF, and infants must be given HBV HBIG.