Current Pediatric Research
International Journal of Pediatrics
Congenital pulmonary adenomatoid malformation: Indication and management of fetal shunting in our center
Molinaro F1, Schneider A2, Talon I2, Sananes N3, Messina M1, Becmeur F1 and Favre R3
1Division of Pediatric Surgery, Department of Medical Science, Surgery and Neuroscience, University of Siena, Italy
2Division of Pediatric Surgery, University of Strasbourg, France
3Division ofgynecology, University of Strasbourg, France
- *Corresponding Author:
- Molinaro F
Division of Pediatric Surgery
Department of Medical Science
Surgery and Neuroscience
University of Siena
Via Val d’Aosta 23, 53035 Monteriggioni (SI) Italy
Tel: +39 3206183601
E-mail: sicamarina121@gmail.com
Accepted on 3rd, June 2019
Objective: Congenital pulmonary adenomatoid malformations (CPAM) are increasingly diagnosed in recent years thanks to the expert hands and the technology of ultrasonography used in antenatal diagnosis. The purpose was to examine a series of cases referred to our fetal-maternal tertiary center for lung disease and to present the natural course, treatment and outcome of antenatally diagnosed cystic lung disease.
Material and Methods: We proposed a retrospective study (October 2003 to September 2016) in a single center. Prenatal and postnatal data of fetus, with suspected CPAM, where analyzed.
Results: We studied 34 cases. One child was excluded post-natally because at birth diagnosis of esophageal duplication was made. Mean gestational age at the diagnosis was 22 weeks (range 21 to 28). Thoracoamniotic shunting was performed in 9 cases. In 8 cases, it was an intra-cystic drain and in 1 case, a pleuroamniotic drain. Mean gestational age for drainage was 25 weeks (range 22 to 28). Mean gestational age at delivery was 38 weeks. All patients underwent postnatal radiological assessment. Anatomical surgical resection was performed in all cases: immediately at birth in 7 symptomatic cases, in the first year of life for 1 case that became symptomatic and 1 patient died immediately at birth due to severe respiratory distress.
Conclusion: Antenatally diagnosed CPAM have an excellent prognosis. We propose invasive prenatal thoracoamniotic shunting in rare cases with unequivocal fetal compromise (i.e., polydramnios and hydrops).
Keywords
Thoracoamniotic shunt, CPAM, Prenatal treatment, Fetal surgery.
Introduction
Congenital lung malformations include a spectrum of rare but clinically significant developmental abnormalities, including congenital pulmonary adenomatoid malformations (CPAM), bronchopulmonary sequestrations (BPS), bronchogenic cysts and congenital lobar emphysema. Many theories relating to the specific etiology of each of these anomalies have been proposed; nevertheless, it is generally agreed that they collectively result from perturbations in lung and airway embryogenesis. Moreover, these clinical entities as well as congenital high airway obstruction syndrome (CHAOS) are likely to have their origin in prenatal airway obstruction, differing only in the gestational age at which obstruction occurs. Antenatal diagnosis of lung malformations increased in recent years with the widespread use of antenatal ultrasound and improved technology [1-4].
CPAM is the most common lung lesion disease. The incidence of CPAM has been estimated to range from 1 in 25,000 to 1 in 35,000 pregnancies. During pregnancy presentation of CPAMs range from fetal hydrops, mediastinal shift to complete in utero regression [5,6]. Postnatal presentation varies from severe respiratory distress to complete lack of symptoms. However, CPAM may not remain asymptomatic throughout life with complications including pneumonia, hemoptysis, pneumothorax, hemothorax and rarely malignancies potentially developing [1,7,8].
Although fetal therapies such as thoracoamniotic shunting can be applied to congenital cyst adenomatoid malformations of the lung diagnosed in utero, there is no clear consensus regarding their indications [9,10]. Our purpose was to evaluate a management policy in which aggressive fetal therapy was restricted to those cases complicated by major polydramnios or hydrops. All other cases were managed conservatively. We reviewed a consecutive series of prenatal management of CPAM to our neighboring tertiary referral center.
Materials and Methods
We performed a retrospective study population (October 2003 to September 2016). We included a series of consecutive patients referred to our tertiary center of fetal medicine, for fetal lung malformations detected at routine ultrasound. CPAM was diagnosed between 21 and 28 gestational weeks (medium: 22GW) (Table 1). For clinical management, the more recent classification into either micro- or macrocystic appearance on ultrasound is more relevant. Macrocystic (Stocker type I) lesions contain at least one cyst >5 mm, whereas microcystic (Stocker type III) lesions show no cyst and appear echogenic. Microcystic lesions tends to regress spontaneously after a growth peak at approximatively 26-28 week’s gestation, but macrocystic lesions generally do not regress, as fluid accumulates in the cyst.
| Case No. | Prenatal Diagnosis (CCAM) |
Gestational age | Size of lesion (emi-thorax) | Mediastinal shift | CVR | PROM |
|---|---|---|---|---|---|---|
| 1 | Type I | 21 w | <50% | no | <1.6 | Yes |
| 2 | Type II | 22 w | <50% | yes | <1.6 | No |
| 3 | Type I | 22 w | >50% | yes | >1.6 | Yes |
| 4 | Type II | 26 w | >50% | no | <1.6 | No |
| 5 | Type I | 22 w | >50% | yes | >1.6 | No |
| 6 | Type III | 25 w | <50% | yes | <1.6 | No |
| 7 | Type III | 22 w | >50% | no | <1.6 | No |
| 8 | Type III | 23 w | <50% | no | <1.6 | No |
| 9 | Type I | 25 w | <50% | no | <1.6 | Yes |
| 10 | Type II | 22 w | <50% | yes | <1.6 | No |
| 11 | Type II | 24 w | >50% | yes | >1.6 | No |
| 12 | Type I | 23 w | >50% | no | <1.6 | No |
| 13 | Type II | 22 w | <50% | yes | <1.6 | No |
| 14 | Type I | 23 w | <50% | no | <1.6 | No |
| 15 | Type II | 23 w | >50% | no | <1.6 | No |
| 16 | Type III | 23 w | <50% | yes | <1.6 | No |
| 17 | Type II | 26 w | >50% | yes | >1.6 | Yes |
| 18 | Type III | 22 w | >50% | yes | >1.6 | No |
| 19 | Type II | 23 w | <50% | no | <1.6 | No |
| 20 | Type II | 23 w | <50% | yes | <1.6 | No |
| 21 | Type II | 26 w | <50% | no | <1.6 | No |
| 22 | Type III | 22 w | <50% | no | <1.6 | No |
| 23 | Type I | 22 w | >50% | no | <1.6 | No |
| 24 | Type I | 25 w | <50% | no | <1.6 | No |
| 25 | Type III | 24 w | >50% | yes | >1.6 | No |
| 26 | Type I | 22 w | <50% | no | <1.6 | No |
| 27 | Type III | 21 w | <50% | no | <1.6 | No |
| 28 | Type III | 22 w | >50% | yes | >1.6 | No |
| 29 | Type I | 21 w | >50% | yes | <1.6 | Yes |
| 30 | Type III | 22 w | >50% | yes | >1.6 | No |
| 31 | Type III | 25 w | >50% | yes | <1.6 | No |
| 32 | Type III | 22 w | >50% | no | <1.6 | No |
| 33 | Type I | 22 w | <50% | no | <1.6 | No |
Table 1. Classification of our series of patients.
To evaluate the size of the lesion, we calculated the percentage of the overall pulmonary area appearing pathological at ultrasound: less than 50% when it was unilateral, 50% with no normal lung on the CPAM side and no mediastinal shift.
When mediastinal shift was observed the size was graded as more than 50% of the thorax. We also considered the CPAM volume ratio (CVR) calculated from the size of the lesion and head circumference (HC): (lengh x height x width x 0.52)/HC. A CVR more than 1.6 is predictive of 80% risk of hydrops. Very large lesions carry a significant risk of causing both pulmonary hypoplasia due to compression of lung tissue and fetal hydrops, probably due to impaired cardiac function as a result of mediastinal shift and compression of lung tissue and fetal hydrops, probably due to impaired cardiac function as a result of mediastinal shift and compression of the vena cava or cardiac tamponade.
Fetal thoracoamniotic shunt was offered in the case of macrocystic lesions with fetal hydrops or signs of evolving hydrops such as ascites, lesions that were either very large (CVR>1.6) or rapidly increasing in size that were associated with polyhydramnios. In the absence of any of these findings, fetuses were followed prospectively with serial ultrasound examinations. We obtained informed consent from the patient before any procedure.
Fetal intervention
We offered thoracentesis or placement of a thoracoamniotic shunt when hydrops developed prior to 32 weeks. This procedure resulted in resolution of both mediastinal shift and hydrops until successful delivery at 37-38 gestational weeks. However, it was important to note that despite successful prenatal cyst decompression, these infants had significant pulmonary hypoplasia and mass effect at birth and potential side-effects due to fetal intervention are induction of premature labor or rupture of the membranes.
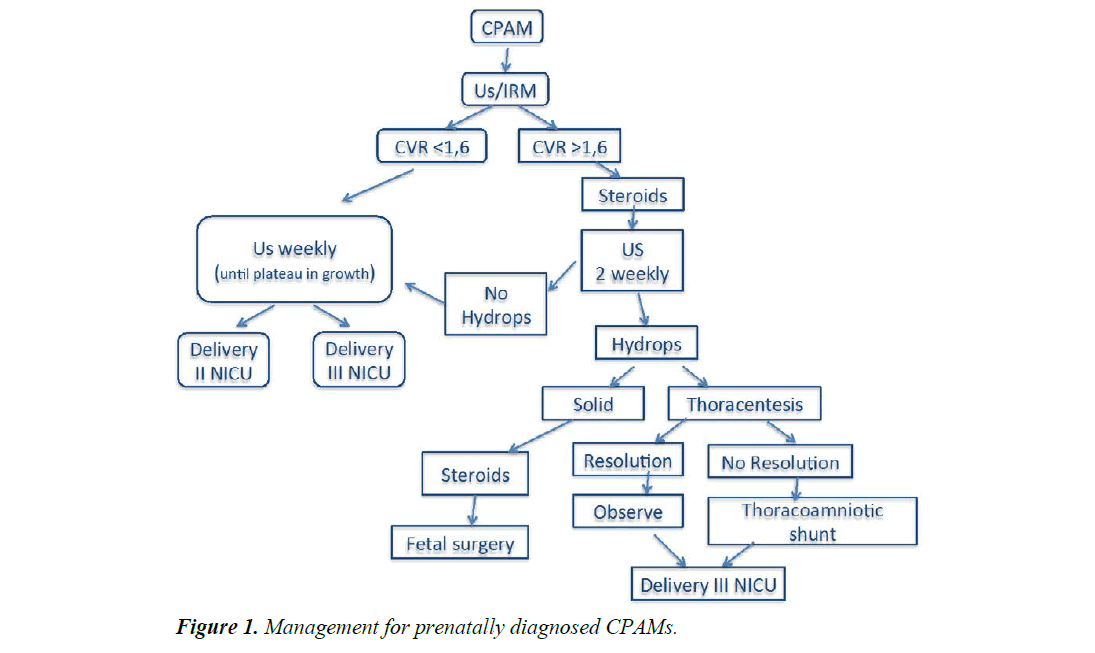
All the uncomplicated cases were treated conservatively. Fetal karyotype was analysed in all cases. Fetal follow-up was monitored with serial prenatal ultrasonography every 1 to 2 weeks (Figure 1).
Fetal shunt technique
In fetal shunt the drainage was inserted through the mother’s abdomen and uterus under echographic control.
The procedure was performed in the hospital. The mother was hospitalized the day before, blood examination and anesthesiologist control was made. The day of the procedure the mother was fasting, and medicaments were done (Atarax and Imidazolam). Under ultrasonographic control and local anesthesia percutaneous thoracoamniotic drainage was inserted. We used a metal cannula that was introduced into the amniotic cavity, through the fetal chest wall into the largest cyst. A double-pigtail catheter (Rocked KCH Fetal Bladder Catheter, London) was inserted through the cannula, with one end in the cyst and the other in the amniotic cavity. The placenta was never traversed.
Two intramuscular doses of betamethasone were given for fetal lung maturation if shunting took place between 24 and 34 weeks’ gestation or subsequently at viability if shunts had been placed at <24 weeks’ gestation. Fetuses were never paralyzed for the procedure.
The mother was discharged the day after previous clinical and ecographic examination and was controlled weekly. Cesarean sections were not routinely performed. All women delivered in our tertiary centre, with neonatalology personnel in attendance. To avoid development of neonatal pneumothoraces, shunt were clamped immediately upon delivery.
Neonatal diagnosis of the mass included chest x-ray and when necessary, computed tomography. Depending of the conditions of the neonate, surgery was performed either as an emergency procedure or in the first year of life. Contraindications for prenatal drainage include: chromosomal abnormality, multiple gestation, associated malformations and the presence of other maternal medical or psychosocial risk factors [9-11].
Results
The mean gestational age at the diagnosis was 22 weeks (range 21 to 28). We classified fetuses according to the Stoker’classification: 11 type I, 11 type II and 12 type III. The malformation was bilateral in one case.
Of 34 cases, we considered 2 different groups: Group A (no prenatal drainage) and Group B (prenatal drainage). Thoracoamniotic shunting was performed in 10 cases, at the mean gestational age of 25 weeks (range 22 to 28). In 9 cases it was a cystoamniotic drain but in 1 case it was a pleuroamniotic drain. In all patients, the size of the lesions decreased substantially.
In the postnatal course, one case of supposed type I CPAM was finally diagnosed as having esophageal duplication and was excluded. Two cases were diagnosed as having a pulmonary sequestration and one case a hybrid CPAM with a sequestration.
Of the 33 cases in which the diagnosis of CPAM was confirmed post-natally, 9 were complicated by fetal hydrops and major polydramnios. The cyst occupied 50% or >50% of the thorax in 16 cases 48% (7 in the group B 43%) and <50% in 17 cases 52% (2 in Group B 11%), mediastinal shift was present in 16 cases 48% (4 in Group B 25%) and premature rupture of the membranes was present in 5 cases 15% (2 in Group B 40%). The mean gestational age (Group A and B) at delivery was 38 weeks (range 25 to 39 weeks).
The cesarean was performed in 11/33 cases at mean gestational age of 39 w (range 25 to 41) (Table 2). There are only 4 cesarean sections among the 9 drained cases. In Group B, respiratory distress at birth required respiratory support in all cases. One patient died. Associated anomalies were found in 2 cases such as pectus excavatum in one case and heart failure in the other case (Table 3).
| Variables | Group A | Group B |
|---|---|---|
| Number of patients | 24 | 9 |
| M/F | 11/13 | 6/4 |
| Hybrid lesion (CCAM+BPS) | 2 | 1 |
| Cesarian delivery | 7 | 4 |
| Mean gestational age at delivery | 38 w | 38 w |
| Death | 4 | 1 |
Table 2. Characteristics of our series of patient: Group A (patient treat conservately) Group B (patient drainated antenatally).
| Case No | Prenatal Diagnosis (CCAM) |
Drain | Delivery | Respiratory Distress | Diagnosis | Surgery | Associated Malformations |
|---|---|---|---|---|---|---|---|
| N° 1 | Type 1 | 22 w | 38 w | Yes | CCAM type 1 (LIR) | 5 Days | No |
| N° 2 | Type 1 | 21 w | 39+6 w | Yes | CCAM type 1 (bilateral) | 2 Days, 3 Months, 5 Years | No |
| N° 3 | Type 3 | 30+5 w | 34 w | Yes | BPS | 1 Day | Heart Failure |
| N° 4 | Type 1 | 28 w | 28 w | Yes | CCAM type 1 (LIR) | 2 Days | No |
| N° 5 | Type 1 | 27 w | 34+5 w | Yes | CCAM type 1 (LIL) | 3 Days | No |
| N° 6 | Type 3 | 28 w | 39+4 w | Yes | CCAM+BPS | 9 Months | No |
| N° 7 | Type 2 | 23 w | 38 w | Yes | CCAM type 2 (LSR) | 1 Day | No |
| N° 8 | Type 1 | 25 w | 38+6 w | Yes | CCAM type 1 | - | - |
| N° 9 | Type 3 | 24 w | 38+2 w | Yes | Death | - | - |
Table 3. Classification of patients treated shunting antenatally.
All children (Group A and Group B) had a postnatal chest x-ray. Surgical treatment was performed in 7 cases in the neonatal period and in 1 case in the first year of life for Group B but in Group A we performed surgical treatment in the neonatal period in 2 cases and in 18 cases in first year of life respectively died at birth 5 patients, 4 in Group A and 1 in Group B, for respiratory distress.
In one bilateral case (Group B) we performed partial atypical resections in three different surgical times. Histopathology revealed isolated CPAM in 30, sequestration in 2 cases and hybrid lesion CPAM type II + sequestration in 1 case. The mean clinical and radiological follow-up was 5 years (between 2 months and 8 years).
Discussion
Antenatal diagnosis of congenital lung malformations has increased in the last decade with rapid advances in imaging technology and increasing use of fetal ultrasonography. The incidence seems to be increasing reporting 1 in 10,000 from Western Australia and 1 in 16,000 from Hong Kong. Probably this is correlated with the increasing antenatal ultrasound screening and improvement in ultrasound technology [3]. Differential antenatal diagnosis of congenital lung malformations includes CPAM, sequestration, bronchogenic cyst and congenital lobar emphysema. In our series, only 3 children were wrongly diagnosed with CPAM on antenatal ultrasound while it was esophageal duplication in 1 case and bronco-pulmonary sequestration in 2 cases. Once the diagnosis of CPAM is suspected, the child could be referred to a tertiary fetus-maternal center where further imaging can be accurately performed and prenatal counseling can be delivered.
The volume of the CPAM is measured and compared to the fetal head circumference. This ratio is known as the CPAM volume ratio (CVR). A CVR of 1.6 indicates a fetus at increased risk for the development of fetal hydrops. The need for shunting is determined not alone by CVR but also by fetal and maternal clinical conditions [12,13].
The basis for considering decompression of cysts in utero is the hypothesis that compression by CPAM is responsible of pulmonary hypoplasia and hydrops. Early hydrops clearly appears as the most important prognostic element. It is thought to be secondary to the extreme mediastinal shift caused by the CPAM, resulting in vena cava and cardiac compression. It was seen also in other space-occupying masses such as congenital diaphragmatic hernia. Also polyhydramnios is frequently correlated with CPAM because fetal swallowing decreased due to esophageal compression by the mass. Another complication of space-occupying thoracic masses would be pulmonary hypoplasia secondary to compression of the remaining tissue [12-15]. Originally, it was suggested that, because of the dismal prognosis for hydropic fetuses with large cystic lung lesions, open fetal surgical resection should be offered. Subsequently, it has become clear that these fetuses can be successfully treated with the much less invasive approach for thoracoamniotic shunting. A recent systematic review showed an improved survival rate of 62% (15/24) in treated hydropic fetuses Vs 3% (1/33) in those untreated (OR, 19.28; 95% CI, 3.7-101) [16].
The role of fetal thoracoamiotic shunt is still controversial for several reasonscand is difficult to define on the base of the literature. Firstly, it may be difficult to predict the evolution of a macrocystic lung lesion (either in increase or decrease in size) and the sequence or timing of development of hydrops. Secondly, there are no randomized studies comparing treatment vs non-treatment in non-hydropic fetuses. Shunting is usually offered only in more severe cases, i.e., those with polyhydramnios, large lesions or severe mediastinal shift, whereas milder cases usually are not treated.
In our center we consider invasive prenatal treatment only in case of unequivocal fetal compromise. We have not performed this procedure for CPAM type III, in which the only therapeutic treatment options were either delivery or open fetal surgery. Although cases eligible for fetal intervention should be carefully selected for the potential risk that this kind of procedure have (i.e., premature labor or premature rupture of membrane) [15-17]. Few studies have reported on macrocystic lung lesions specifically. Microcystic and macrocystic lesions seem to differ significantly in their growth patterns during pregnancy and in their prognosis. Whereas a decrease in size during the third trimester has been reported in combined groups of macro- and microcystic CPAMs, it has been suggested that macrocystic lesions may have a tendancy to invrease in size [18,19].
In recent literature, there is a limited experience of percutaneous laser ablation to treat multicystic lesions. Although tumor size decreased, the hydrops worsened and fetal death occurred [20]. Moreover other study in Korean population found that percutaneous injection of OK-432, a sclerosing agent, is also a good choice to treat these fetuses [21].
In our experience, shunting appeared useful in several cases. The advantages and potential complications of invasive management should be carefully weighed with regard to gestational age and the risk of premature labor. Perinatal management has much to gain from the precise prenatal diagnosis of CPAM, which allows for in utero therapy, for emergency surgery after delivery, and for appropriate follow up of less severe cases.
We treated symptomatic CPAMs in the first year of life with a good outcome. Asymptomatic CPAMs were observed and treated when they became symptomatic. We have not so long follow-up range 1 month to 8 years and we have no complications. The limitations of our study are that the data were retrospective and observational. Only shunted cases were evaluated and there was no control group, although we suggest that observing a control group of hydropic fetuses would be unethical. In the literature creasing evidence of complications of CPAMs including rapid expansion of CPAM during life and also Nars et al. from Toronto recently reported 4% incidence of pleuro-pulmonary blastoma in asymptomatic lung lesion non-treated. Rabdomiosarcoma have also been found to originate from CPAM.
Conclusion
We agree with recent literature that thoracoamniotic shunting should be offered for all hydropic fetuses with large macrocystic lung lesions and we suggest that it should be considered also for non-hydropic fetuses if the predicted risk of developing hydrops is high. Specifically, this includes fetuses with very large lung lesions that increase in size rapidly or are associated with polyhydramnios. We suggest that fetuses with large macrocystic lung lesions should be referred to a regional fetal medicine center for thorough evaluation and fetal intervention as appropriate to limit the risks linked to inexperienced surgeons. In our experience, a very good fetal outcome with a low complication rate can be achieved only in expert hands.
References
- Azizkhan RG, Crombleholme P. Congenital cystic lung disease: Contemporary antenatal and postnatal management. Pediatric Surg. 2008; 24: 643-657.
- Mendeloff EN. Sequestration, congenital cyst adenomatoid malformations, and congenital lobar emphysema. Thorac Cardiov Surg. 2004; 16: 209-214.
- Raychaudhuri P, Pasupati A. Prospective study of antenatally diagnosed congenital cystic adenomatoid malformations. Pediatr Surg. 2011; 27: 1159-1164.
- Langston C. New concepts in the pathology of congenital lung malformations. Seminars in Pediatric Surg. 2003; 12: 17-37.
- Witlox RS, Lopriore E. Neonatal outcome after prenatal interventions for congenital lung lesions. Early Human Development. 2011; 87: 611-618.
- Kunisaki SM, Barnewolt CE. Large fetal congenital cyst adenomatoid malformations: Growth trends and patients survival. J Pediat Surg. 2007; 42: 404-410.
- Adzik NS, Harrison MR. Fetal lung lesions: Management and outcome. Am J Obstet Gynecol 1998; 179: 884-889.
- Khosa JK, Leong SL. Congenital cystic adenomatoid malformation of the lung: Indications and timing of surgery. Ped Surg Int. 2004; 20: 505-508.
- Dommergues M, Louis-Sylvestre C. Congenital adenomatoid malformation of the lung: When is active fetal therapy indicated? Am J Obstet Gynecol. 1997; 177: 953-958.
- Azizkhan RG, Crombleholme TM. Congenital cystic lung disease: contemporary antenatal and postnatal management. Ped Surg Int. 2008; 24: 643-657.
- Le Nuè R, Molinaro F. Surgical management of congenital chylothorax in children. Eur J Ped Surg. 2010; 20: 307-311.
- Gregory CL, Wringht J. A review of fetal thoracoamniotic and vescicoamniotic shunt procedures. J Obstet Gynecol Neonatal Nurs. 2012; 41: 426-433.
- Dumez Y. Madelbrot L. Prenatal management of congenital cystic adenomatoid malformation of the lung. Journal of Ped Surg. 1993; 28: 36-41.
- Harrison MR, Scott Adzick N. Antenatal intervention for congenital cystic adenomatoid malformation. Lancet. 1990; 336: 965-967.
- Hourrier S, Salomon LJ. Prenatal diagnosis and management of foetal lung lesions. Revue des Maladies Respiratoires. 2011; 28: 1017-1024.
- Knox EM, Kilby MD. In-utero pulmonary drainage in the management of primary hydrothorax and congenital cystic lung lesion: A systematic review. Ultrasound Obstet Gynecol. 2006; 28: 726-734.
- Schrey S, Kelly EN. Fetal thoracoamniotic shunting for large macrocystic congenital cystic adenomatoid malformations of the lung. Ultrasound Obstet Gynecol. 2012; 39: 515-520.
- Crombleholme TM, Coleman B. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatric Surg. 2002; 37: 331-338.
- Wilson RD, Hedrick HL. Cystic adenomatoid malformation of the lung: review of genetic, prenatal diagnosis, and in utero treatment. Am J Med Genet A. 2006; 140: 151-155.
- Bruner JP, Jarnagin BK. Percutaneous laser ablation of fetal congenital cyst adenomatoid malformation: Too little, too late? Fetal Diagn Ther. 2000; 15: 359-363.
- Min JY, Won HS. Intrauterine therapy for macrocystic congenital cystic adenomatoid malformation of the lung. Obstet Gynecol Sci. 2014; 57: 102-108.