Current Pediatric Research
International Journal of Pediatrics
Causes of peripheral hypotonia in infant and children attending Assiut university children hospital.
Eman Fathalla Gad1, Safiea Abd El-Fattah El-Deeb1, Abeer Hussien2, Azza Abouzeid1*
1Department of Pediatrics, Assiut University, Assiut, Egypt
2Department of Radiology, Assiut University, Assiut, Egypt
- Corresponding Author:
- Azza Abouzeid
Department of Pediatrics, Assiut University, Assiut, Egypt
E-mail: docazza79@gmail.com
Received: 04 February, 2022, Manuscript No: AAJCP-22-53366; Editor assigned: 07 February, 2022, PreQC No: AAJCP-22-53366 (PQ); Reviewed: 15 March, 2022, QC No: AAJCP-22-53366; Revised: 21 March, 2022, Manuscript No: AAJCP-22-53366 (R); Published: 29 March, 2022. DOI:10.35841/0971-9032.26.3.1279-1282.
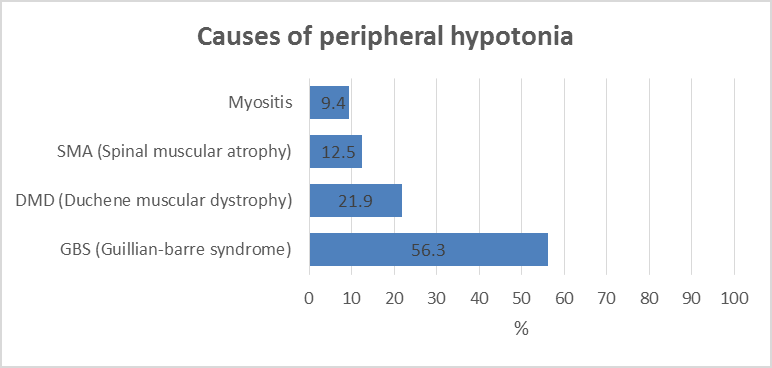
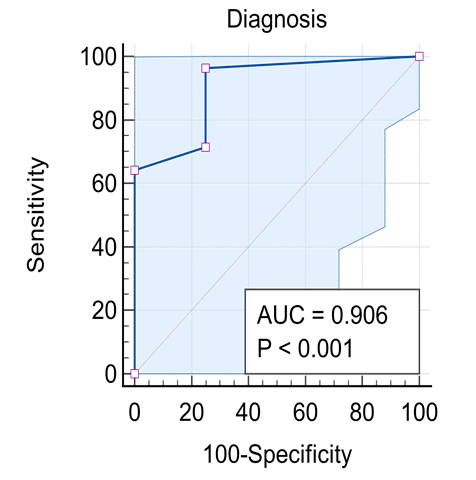
Background: Diseases in the peripheral motor unit are common in children. The term peripheral hypotonia covers disorders of the neuromuscular unit other than causes in the Central Nervous System (CNS). This includes the peripheral motor unit with its four components: Anterior horn cell in the spinal cord, its axons with other axis forming the peripheral nerve (including the cranial nerves) and the neuromuscular junction with the muscle fibers innervated by a single motor neuron. The aim of this work is to give a profile of the causes of peripheral hypotonia in infants and children attending Assiut University Children Hospital. Patients and Methods: 32 patients aged over one month to 14 years were assessed clinically and had creatine kinase enzyme measured, as well as Nerve Conduction (NC) and Electromyography (EMG), together with muscle ultrasonography done. Results: Cases had Guillan-Barre Syndrome (GBS) in 56.3%, Duchene Muscular Dystrophy (DMD) in 21.9% and Spinal Muscular Atrophy (SMA) in 12.5%, and myositis in 9.4%. Ultrasonography showed sensitivity in the diagnosis of peripheral hypotonia in 96.43% and specificity of 75%, with positive and negative predictive values as 87.5% and 12.5% respectively. Conclusion: For neurological cases, hope is present with early discovery of spinal muscular atrophy in newborns by screening with ultrasonography with new treatment by gene therapy because of higher sensitivity and predictive value. Recommendations: A future study in peripheral hypotonia in pediatrics is recommended with a bigger number of cases to support this work.
Keywords
Peripheral, Hypotonia, Children.
Introduction
Diseases of the peripheral motor unit are common in children [1]. Because some of them are fatal and now with early discovery of this disease and early treatment can save newborn by screening in suspected cases with isolated neonatal peripheral hypotonia, in absence of hypoxia and central nervous system causes. This work aims to study the causes of peripheral hypotonia in infants and children attending Assiut university children hospital.
Patients and Methods
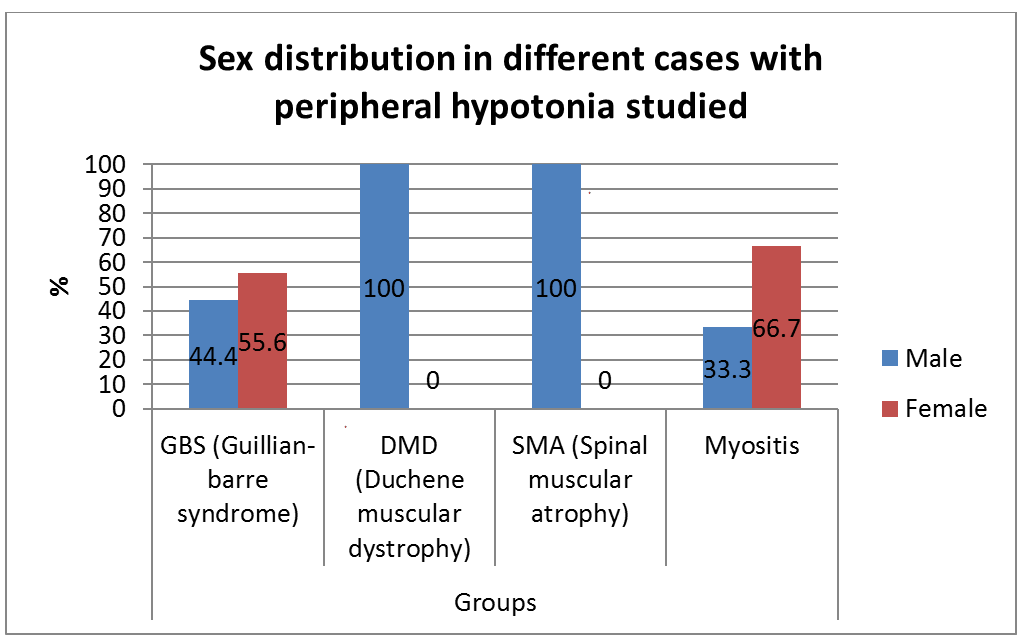
A prospective, descriptive study included 32 infants and children with peripheral hypotonia attending neurology clinic at Assiut university children hospital, 20 males, and 12 females. Their ages ranged from 4 months to 14 years. The ethical review committee, faculty of medicine Assiut university. They all had a full clinical and neurological examination done. The following investigations were done in all patients: creatine kinase, nerve conduction, electromyography, and muscle ultrasonography study [2].
Results
Our results are shown in Figures 1-5 and Table 1.
| Diagnosis (n=32) | EMG | MS US | NC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| GBS (Guillian-Barre Syndrome) | 1 | 5.5 | 17 | 94.4 | 18 | 100 | 0 | 0 | 17 | 94.4 | 1 | 5.5 |
| DMD (Duchene Muscular Dystrophy) | 7 | 100 | 0 | 0 | 7 | 100 | 0 | 0 | 0 | 0 | 7 | 100 |
| SMA (Spinal Muscular Atrophy) | 4 | 100 | 0 | 0 | 2 | 50 | 2 | 50 | 1 | 25 | 3 | 75 |
| Myositis | 3 | 100 | 0 | 0 | 2 | 66.6 | 1 | 33.3 | 0 | 0 | 3 | 100 |
Table 1. Shows the statistical agreement between the neuro-electrical studies and ultrasonography study n=32.
Discussion
With the criteria of diagnosis peripheral hypotonia being absent or depressed tendon reflexes, failure of movements, fasciculation and muscle atrophy [3], in the present study, guillan-barre syndrome is the commonest cause of peripheral hypotonia (Figure 1). Gillian-Barre Syndrome constitutes one of the serious emergencies. This finding agrees with, who stated that GBS has been typically linked to inflammatory destruction of the myelin sheaths of the peripheral nerves and roots; termed acute inflammatory demyelinating polyradiculoneuropathy. This is commonest in the age group 2-15 years [4]. In the present study, GBS occurs in cases reached 2-14 years. This is also in agreement with Ashrafi et al. who added that GBS had different clinical variants regarding the types of nerve injuries (sensory, motor, sensory and motor, autonomic or cranial); the modes of nerve injuries (demyelinating or axonal).
It can present with loss of consciousness with paralysis and respiratory failure in a few days of the onset. The exact pathophysiology of GBS remains insufficiently understood, it is believed that both cellular and humeral components of the immune system are involved. The infiltration of lymphocytic mononuclear cells surrounding the peripheral nerves and nerve roots occurs. Furthermore, the presence of autoantibodies against the myelin sheaths of the peripheral nerves has been observed in the other forms of GBS. So that the typical to albuminous dissociation with elevated levels of CSF proteins but normal cell count in CSF has been reported. In addition, previous upper respiratory tract infections or gastroenteritis has been described to occur two weeks before the onset of GBS [5]. The relationship between infection and GBS is worthy of note, as the present study has been conducted in the COVID-19 pandemic and we observed that man of cases are admitted to ICU and many of them needed assisted ventilation. In this respect, our results with an agreement with Ashrafi et al.
Unlike autoimmune diseases, observed that steroids were not effective in the alleviation of muscle weakness in GBS. The mainline of therapy in GBS is IVIG and/or plasmapheresis [6]. Duchene Muscular Dystrophy (DMD) was the next in frequency to GBS in this study (Figure 1). Muscular dystrophies are caused by mutations in genes responsible for the production of proteins essential for contractile cytoskeletal signaling or enzymatic function within the muscle fibers and the extracellular matrix. DMD is a primary myopathy with a genetic basis, a progressive course, and degeneration with the death of muscles fibers that occurs at some stages of the disease [7]. Finkel et al. also stated that in DMD usually there is intellectual impairment, muscle hypertrophy with pain in the calf, and subsequent muscle replacement with fat and fibrosis. In this study, both in GBS and DMD, the ultrasonography showed statistical agreement with a clinical, electromyography, nerve conduction as well as with creatine kinase level in counted 100% of the cases (Table 1).
The increase in the muscle echogenicity is judged by comparing the muscle echogenicity to echogenicity with surrounding structures, e.g. subcutaneous tissue, which should be of similar echogenicity. Assessment of the severity of muscle affection can also be done by comparing muscle echogenicity to bone echo [8]. Those authors reported that ultrasonography sensitivity ranged from 70% to 91% of the cases. They also added that muscle ultrasonography is also useful in cases with spinal muscular atrophy and myositis. Albyada et al. added that ultrasonography may detect pathological phenomenons (fasciculation and even fibrillations in cases with peripheral hypotonia), especially shown in cases with myopathy and myositis associated with raised CPK enzyme.
Spinal Muscular Atrophy has been encountered in 12.5% of the cases in this study (Figure 1). Unlike myopathy, in cases with SMA, the ultrasonography shows an inhomogeneous increase in echogenicity, with a moth-eaten appearance or completely white muscle with severe atrophy [8]. In SMA, the most common autosomal recessive condition leads to death in children, as stated by Hewedi et al. In the present study, 50% of our cases ended fatally with respiratory failure. Maria et al. reported that achieving an early diagnosis is very important in children with SMA not only for genetic counseling, and for implementation of the diagnosis-specific standard of cases and treatment. The recent advent of new therapeutic options, e.g. SHAM and NUSINERSEN pre-symptomatic therapy, has further increased the need to confirm the diagnosis as early as possible [9]. In the present study, ultrasonography was in agreement with clinical and electro neurological studies. In fact, this encouraged us to recommend the neonatal screening of peripheral hypotonia in a neonate without a central cause, e.g. hypoxia, and without being preterm with a unique form of respiratory distress [10].
Conclusion
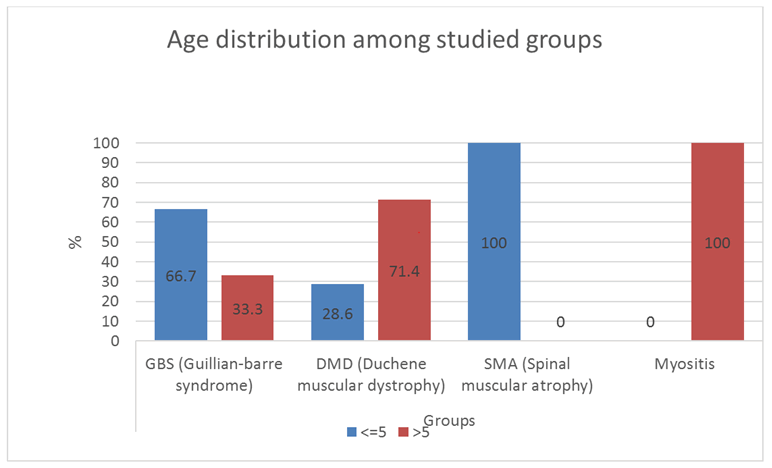
In the present study, peripheral hypotonia was present in 9.4% of myositis. The age ranged from 8 to 14 years. This agrees with Giacomo et al. who showed that these cases occurred in school-aged children with symmetric lower limb pain and calf tenderness with sudden walking abnormalities 3-4 days after an initial viral illness. This condition must be differentiated from DMD, having similar ages, by the normal tendon reflexes by the normal nerve conduction study. The ultrasonography was reported in 1/3 of our myositis cases to be normal, but this is maybe because ultrasonography was performed after improvement had occurred in these cases, despite raised CPK enzyme. But we hope for more use of ultrasonography in cases with chronic myositis, polymyositis, connective tissue diseases, and dermatomyositis, a better agreement between ultrasonography and electromyography will be obtained in the future study.
Recommendations
•Ultrasonography screening for full-term neonates with peripheral hypotonia without hypoxia to be done together with taking the blood samples for the neonate for CPK enzyme level estimation.
•Future studies on peripheral hypotonia in pediatrics with bigger number of cases is recommended to support this present work.
References
- Regina Célia BD. Hypotony in children. Residência Pediátrica 2018; 39(1): 43. [Crossref][Google Scholar]
- Harvey B, Sarnat. Evaluation and investigation of neuromuscular disorders. Nelson text book of pediatric 2021; 625(2): 12606-12627.
- Hewedi KM, Mohie El-Din TM, Mohamed SY. Evaluation of children with peripheral hypotonia by electroneuromyography. Al-Azhar Assiut Medical Journal 2016; 14: 134-139. [Crossref][Google Scholar]
- Malcolm Rabie, Stephen Ashwal, Yoram Nevo. Inflammatory neuropathies. Swaiman's pediatric neurology text book 2018; 143: 1086-1091.
- Ashrafi MR, Ariadokht M, Alireza R, et al. Clinical characteristics and electro diagnostic features of Guillain-Barr´e syndrome among the pediatric population. J Child Neurol 2020; 35(7): 448-455. [Crossref][Google Scholar][Indexed]
- Solana R, Luis Garcia-Maria M, Maria Reyes-Varela D, et al. Clinical severity and associated complications in pediatric patients with Guillian-Barre syndrome. Revista Mexicana de Neurociencia 2020; 21(1): 15-26. [Crossref][Google Scholar]
- Richard S, Finkel A. Clinical assessment of pediatric neuromuscular disorders, Swaiman's pediatric neurology text book 2018; 137: 1044-1050.
- Albayda J, Nens van A. Diagnostic value of muscle ultrasound for myopathies and myositis. Current Rheumatology Reports 2020; 22: 82. [Crossref][Google Scholar][Indexed]
- Maria Carmela P, Giorgia C, Beatrice Berti, et al. Diagnostic journey in spinal muscular atrophy: Is it still an odyssey? PLoS ONE 2020; 15(3): e0230677. [Crossref][Google Scholar][Indexed]
- Sally L, Chriselle H, Alison DA. A mixed methods exploration of families' experiences of the diagnosis of childhood spinal muscular atrophy. Eur J Hum Genet 2015; 23(5):575-80. [Crossref][Google Scholar][Indexed]